3D Systems receives FDA approval for new 3D printed surgical
US-based 3D printer manufacturer 3D Systems has received 510(k) clearance from the Food and Drug Administration (FDA) for its 3D printed Total Ankle pre-surgical planning and instrument set.
The new offering is personalized to meet specific patient needs and ensure accurate implant alignment and sizing for ankle replacement surgery. According to 3D Systems, Total Ankle saves surgeons time in the operating theatre and ensures accuracy while performing fewer steps than with standard instruments.
The 3D printed guides can be paired with global medical technology company Smith+Nephew’s SALTO TALARIS Total Ankle Prosthesis and CADENCE Total Ankle System. They are being engineered and produced using 3D Systems’ in-house Virtual Surgical Planning (VSP) solutions.
This new FDA clearance expands the company’s growing medical device portfolio, which has already supported over 175,000 surgical cases. The firm hopes expanding the adoption of Total Ankle will allow it to capitalize on the growing global orthopedic devices market, which is expected to reach $5.3 billion by 2032.
“3D Systems is a recognized leader in supporting the medical device industry and surgeon community with early adoption in craniomaxillofacial procedures,” commented Gautam Gupta, general manager and senior vice president of medical devices, 3D Systems.
“Through our collaboration with an industry leader such as Smith+Nephew, we leveraged our collective expertise in orthopedics to develop an end-to-end solution for total ankle replacements that is helping surgeons perform surgeries more efficiently.”
 3D printed Salto Optech Tibia Guide. Image via 3D Systems.
3D printed Salto Optech Tibia Guide. Image via 3D Systems.
FDA-clearance for 3D printed surgical guides
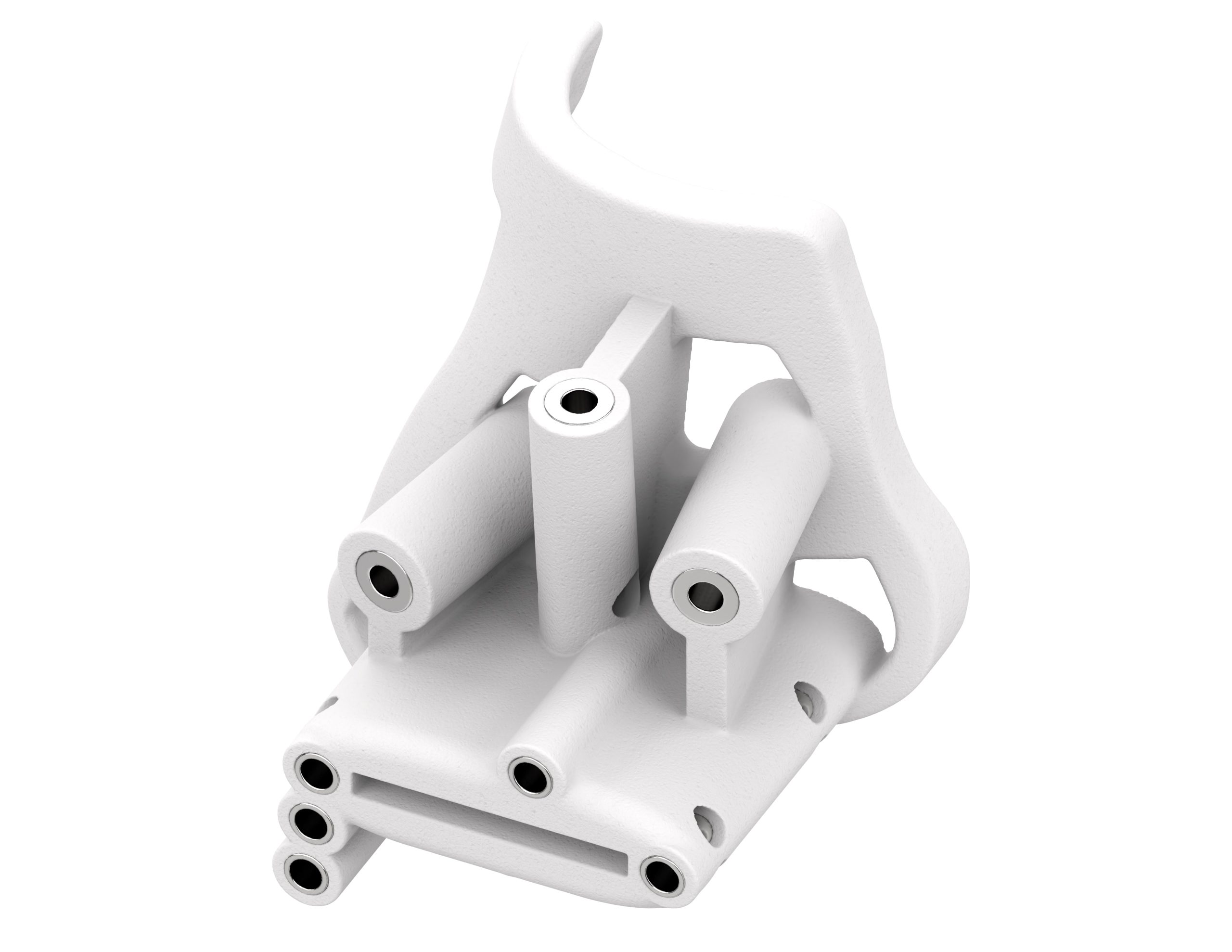
3D System’s 3D printed Total Ankle devices enable surgeons to prepare the bony anatomy of the tibia and talus for the placement of implants designed to restore proper ankle joint function.
They reportedly allow medical professionals to perform these operations in fewer procedural steps, saving time and reducing exposure to intraoperative X-ray radiation compared to standard non-patient-specific instruments.
The company’s VSP surgical planning workflow combines expertise from biomedical engineers with its Selective Laser Sintering (SLS) 3D printers and DuraForm ProX PA materials.
To date, 3D Systems has fabricated over 2 million medical device implants and supported more than 100 FDA-cleared and CE-marked devices. The company is expanding its expertise beyond 3D printed craniomaxillofacial devices to other skeletal applications, including large joints. It has also identified 3D printing potential in trauma environments where quality and response time are vital.
Ben Johnson, 3D Systems’ vice president of portfolio & regulatory, calls VSP a “differentiator in the market.” He added that VSP was instrumental in the success of the Total Ankle program within a short timeframe.
According to Mark McMahan, vice president of marketing, global orthopaedics at Smith+Nephew, Total Ankle can help “transform the way healthcare professionals approach surgical precision.” He added that the 510(k) certified products provide “unparalleled efficiency and accuracy,” enhancing surgeon and patient outcomes.
Last week, 3D Systems and Smith+Nephew showcased their 3D printed ankle surgery guides at the 2024 American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting.
 3D printed Cadence Optech Talus Guide. Image via 3D Systems.
3D printed Cadence Optech Talus Guide. Image via 3D Systems.
Take 10 seconds to tell us the impact of this news using the block below. Make sure you click submit!
3D printing improves surgical outcomes
Thanks to its ability to quickly fabricate personalized medical devices, 3D printing is being increasingly adopted to optimize surgical procedures.
Earlier this year, 3D printer manufacturer Stratasys announced that its J5 MediJet 3D printer is being used by the University Hospital Birmingham (UHB) to 3D print personalized surgical guides used in craniomaxillofacial tumor surgery.
Tailored to match patient-specific requirements, the cutting guides reportedly reduce surgery time by up to three hours. Previously, these devices had to be made by cutting and bending pieces of metal by hand during operating procedures. The hospital’s surgical team can now use patient scans to 3D print the guides at resolutions of 150 microns.
Elsewhere, digital services company Ricoh USA recently launched the Ricoh 3D for Healthcare Innovation Studio. The new point-of-care additive manufacturing facility is based at Atrium Health Wake Forest Baptist, a medical center in North Carolina.
The facility allows clinicians to develop, design and 3D print FDA-approved anatomic models on-site. These patient-specific devices can then be used for surgical planning and patient education.
According to Ricoch, hospital-based 3D printing can reduce operating times, lower medical costs, improve diagnostic support, and alleviate FDA-compliance concerns. Surgeons using 3D printed anatomical models have reportedly achieved operating time savings of 62 minutes, and a 7.8% reduction in operative time.
Nominations are now open for the 2024 3D Printing Industry Awards.
What does the future of 3D printing hold?
What near-term 3D printing trends have been highlighted by industry experts?
Subscribe to the 3D Printing Industry newsletter to keep up with the latest 3D printing news.
You can also follow us on Twitter, like our Facebook page, and subscribe to the 3D Printing Industry Youtube channel to access more exclusive content.
Featured image shows a 3D printed CADENCE Optech Tibia Guide. Image via 3D Systems.