Mental Health Treatment Providers
Mental Health Treatment Market Overview
Mental health treatment providers’ products include a variety of tools designed to support mental well-being. These products range from pharmaceuticals such as antidepressants and mood stabilizers to digital therapeutics like apps offering CBT and mood tracking.
Moreover, they also encompass self-help resources, including books and guides, wellness tools such as guided meditation recordings, and support platforms providing access to online communities and peer counseling.
Additionally, educational materials, such as brochures and online courses, help individuals understand mental health conditions and treatment options. These products complement professional care and enhance self-management of mental health.
Market Drivers
The mental health treatment product market is driven by increased awareness and reduced stigma around mental health issues, a rising prevalence of disorders, and technological advancements such as digital health apps and teletherapy platforms.
Growth is further supported by increased investment in research, the integration of mental health into primary care, demand for personalized care, and favorable regulatory policies. These factors collectively fuel the expansion and innovation within the market.
Market Size
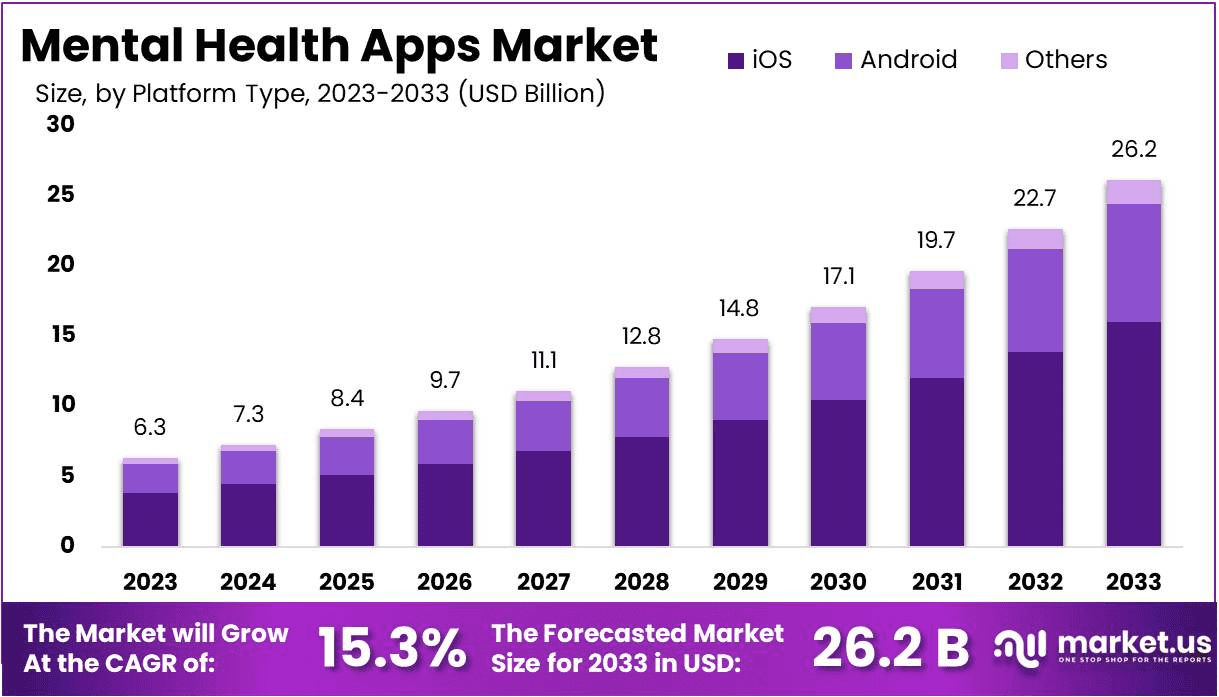
The mental health apps market is projected to reach approximately USD 26.2 billion by 2033, up from USD 6.3 billion in 2023, with a CAGR of 15.3% from 2024 to 2033.
List of Major Companies
These are the top ten companies providers operating in the Mental Health Treatment Market:
Abbott
Company Overview
| Establishment Year | 1888 |
| Headquarter | Green Oaks, Illinois, U.S. |
| Key Management | Robert B. Ford (Chairman & CEO) |
| Revenue (US$ Bn) | $ 40.1 Billion (2023) |
| Headcount | ~ 114,000 (2023) |
| Website | http://abbott.com/ |
About Abbott Laboratories
Abbott Laboratories has been actively expanding its footprint in the mental health treatment providers’ products sector, with a significant focus on developing advanced therapies for treatment-resistant depression (TRD).
A notable development is their initiation of the TRANSCEND clinical trial to evaluate their deep brain stimulation (DBS) system for TRD.
This condition affects approximately 2.8 million Americans annually and often fails to respond to conventional treatments.
This system, which has received Breakthrough Device designation from the FDA, works by delivering targeted electrical pulses to specific brain areas to alleviate symptoms.
This trial underscores Abbott’s commitment to addressing complex mental health issues through innovative, technology-driven solutions and could potentially offer a new lifeline to those with this challenging condition.
Geographical Presence
Abbott Laboratories operates globally across North America, Latin America, Europe, Asia-Pacific, and the Middle East and Africa.
In North America, it focuses on diagnostics, nutrition, and medical devices in the U.S. and Canada. In Latin America, key markets include Brazil, Mexico, Argentina, Chile, and Colombia.
In Europe, Abbott is active in the UK, Germany, France, Italy, Spain, and Eastern Europe. Its Asia-Pacific operations are prominent in China, India, Japan, Australia, and New Zealand.
In the Middle East and Africa, Abbott serves South Africa, the UAE, Saudi Arabia, and other countries, aiming to enhance its healthcare solutions across these regions.
Recent Developments
- In September 2024, Abbott launched the TRANSCEND trial to assess the effectiveness of its deep brain stimulation system for treating treatment-resistant depression (TRD).
- In August 2024, Abbott expanded its Pure Bliss by Similac line with new organic, European-made infant formulas.
Merck
Company Overview
| Establishment Year | 1891 |
| Headquarter | Rahway, New Jersey, U.S. |
| Key Management | Robert M. Davis (Chairman, President and CEO) |
| Revenue (US$ Bn) | $ 60.1 B (2023) |
| Headcount | ~ 72,000 (2023) |
| Website | http://merck.com/ |
About Merck
Merck & Co., Inc. has been active in the mental health treatment providers/sector, specifically focusing on a novel schizophrenia treatment with MK-8189, a phosphodiesterase 10A (PDE10A) inhibitor, currently in Phase 2b development.
This development is part of a collaboration with Royalty Pharma, with co-funded development costs highlighting a strategic approach to funding and resource allocation in mental health innovations.
Recent developments by Merck include the acquisition of companies such as Caraway Therapeutics and Prometheus Biosciences, which align with their strategy to strengthen their pipeline across various therapeutic areas.
Geographical Presence
Merck & Co., Inc., known as MSD outside North America, operates globally and has a significant presence in various regions.
Headquartered in Kenilworth, New Jersey, the company has key operations in Canada and major European markets like Germany and France while expanding in Central and Eastern Europe.
In Asia-Pacific, Merck is active in Japan, China, South Korea, India, and Australia. The company also has a strong foothold in Brazil and Mexico in Latin America, with growing influence in Argentina, Chile, and Colombia.
In Africa, Merck is established in South Africa and is expanding throughout the continent. This global reach supports Merck’s strategy to advance healthcare through its comprehensive portfolio of pharmaceuticals and vaccines.
Recent Developments
- In August 2024, Merck agreed to acquire CN201, a novel bispecific antibody in clinical development for treating B-cell-related diseases.
- In August 2024, the European Commission approved Merck’s Winrevair (sotatercept) for use in combination therapy for adults with functional class II or III pulmonary arterial hypertension.
GSK
Company Overview
| Establishment Year | 2000 |
| Headquarter | London, England, UK |
| Key Management | Emma Walmsley (CEO) |
| Revenue (US$ Bn) | $ 37.7 Billion (2022) |
| Headcount | ~ 70,000 (2024) |
| Website | https://www.gsk.com/ |
About GSK
GSK plc has made significant strides in the mental health treatment providers/sector, focusing on innovative approaches to drug development and patient care.
A recent highlight is their progress with dostarlimab (Jemperli), a monoclonal antibody initially approved for endometrial cancer, which is now being explored for other solid tumors in various stages of clinical development.
This expansion reflects GSK’s strategy to leverage existing drugs for broader therapeutic applications, enhancing their mental health treatment portfolio.
Alongside this, GSK continues to enhance its pipeline through targeted acquisitions, such as the recent purchase of Bellus Health, aimed at bolstering its capabilities in dealing with respiratory conditions potentially linked to mental health issues.
Geographical Presence
GSK plc, headquartered in Brentford, UK, operates on a global scale with a significant presence across multiple regions.
In Europe, GSK maintains operations in the UK and major Western European countries such as Germany, France, Italy, and Spain.
In North America, the company is prominent in the United States and Canada. Its Asia-Pacific footprint includes China and Japan, with additional operations in Australia, India, and South Korea.
In Latin America, GSK is active in Brazil and Mexico, as well as other countries in the region. The company also has a notable presence in South Africa and various other African nations.
GSK’s broad geographical reach supports its global strategy, enabling it to address diverse market needs and drive innovation across its pharmaceutical, vaccine, and consumer healthcare segments.
Recent Development
- In August 2024, GSK received approval from Japan’s Ministry of Health for Nucala (mepolizumab) to treat chronic rhinosinusitis with nasal polyps in adults.
- In August 2024, GSK received FDA approval for Jemperli (dostarlimab) in combination with chemotherapy to treat adults with advanced or recurrent endometrial cancer.
Novartis
Company Overview
| Establishment Year | 1996 |
| Headquarter | Basel, Switzerland |
| Key Management | Vasant Narasimhan (CEO) |
| Revenue (US$ Bn) | $ 45.4 Billion (2023) |
| Headcount | ~ 76,057 (2023) |
| Website | https://novartis.com/ |
About Novartis
Novartis AG has been actively enhancing its presence in the mental health treatment providers/sector, particularly focusing on addressing neuropsychiatric disorders with innovative therapeutic solutions.
A notable development is their ongoing advancement of MIJ821, a treatment for resistant depression, which underscores their commitment to tackling challenging mental health conditions.
This initiative is part of Novartis’ broader strategy to innovate in neuroscience, as highlighted by their acquisition of Cadent Therapeutics.
The acquisition has enriched its pipeline with promising clinical-stage projects for schizophrenia and movement disorders, demonstrating Novartis’ strategy to expand and deepen its neuroscientific research and development capabilities.
Geographical Presence
Novartis AG, based in Basel, Switzerland, has a robust global footprint. In Europe, its operations span Germany, France, Italy, Spain, the UK, and Switzerland.
North American activities are concentrated in the US and Canada, with a key R&D center in Cambridge, Massachusetts. In Latin America, Novartis operates in Brazil, Mexico, Argentina, Chile, and Colombia.
The Asia-Pacific region, including China, Japan, India, Australia, South Korea, and Singapore, is a major growth focus.
In the Middle East and Africa, the company targets South Africa, Saudi Arabia, the UAE, and Egypt. Novartis supports its global strategy through a network of regional offices and R&D centers.
Recent Development
- In September 2024, Novartis began building two new radioligand therapy manufacturing facilities in the US.
- In August 2024, Novartis agreed to sell its molecular imaging business to Siemens Healthineers for $223 million.
Johnson-n-Johnson
Company Overview
| Establishment Year | 1886 |
| Headquarter | New Brunswick, New Jersey, U.S. |
| Key Management | Joaquin Duato (CEO) |
| Revenue (US$ Bn) | $ 85.1 B (2023) |
| Headcount | ~ 134,400 (2023) |
| Website | https://www.jnj.com/ |
About Johnson & Johnson
Johnson & Johnson, with a legacy in neuroscience dating back to the 1950s, has been actively involved in the mental health treatment providers/sector.
Through its Janssen Pharmaceuticals subsidiary, the company offers a range of medications for mental health conditions and is exploring new treatments.
Recent efforts include a major initiative in Rwanda, where Johnson & Johnson is working with the government to enhance mental health services and integrate mobile technology for healthcare worker training.
This initiative reflects the company’s broader commitment to developing scalable, sustainable healthcare models and reducing mental health stigma through innovative technology and community-based solutions.
Geographical Presence
Johnson & Johnson, headquartered in New Brunswick, New Jersey, maintains a vast global presence across multiple continents.
In North America, it operates extensively in the United States and Canada. In Europe, the company has significant operations in the United Kingdom, Germany, France, Italy, and Spain.
Its presence in Asia-Pacific includes major markets like China, Japan, India, and Australia. In Latin America, Johnson & Johnson is active in Brazil and Mexico, while in the Middle East and Africa, it operates in South Africa, the UAE, and Saudi Arabia.
This broad geographical reach enables the company to address diverse healthcare needs and leverage growth opportunities worldwide effectively.
Recent Developments
- In August 2024, Janssen-Cilag International received European Commission approval for BALVERSA to treat adults with unresectable or metastatic urothelial carcinoma (mUC) who have susceptible FGFR3 genetic mutations.
- In August 2024, Johnson & Johnson acquired V-Wave, a heart failure implant company, for US$1.7 billion.
Medtronic
Company Overview
| Establishment Year | 1949 |
| Headquarter | Dublin, Ireland |
| Key Management | Geoff Martha (CEO) |
| Revenue (US$ Bn) | $ 32.3 Billion (2024) |
| Headcount | ~ 95,000 (2024) |
| Website | https://www.medtronic.com/ |
About Medtronic
Medtronic plc has been actively enhancing its offerings in the healthcare technology sector, with recent developments emphasizing its commitment to innovative treatments.
Notably, Medtronic received FDA approval for its Percept™ RC Deep Brain Stimulation (DBS) system, marking a significant advancement in the treatment of neurological disorders, potentially including mental health conditions that benefit from neuromodulation therapies.
This system represents a forward leap in applying technology to manage and potentially alleviate symptoms related to mental health through precise neural stimulation.
Geographical Presence
Medtronic plc, headquartered in Dublin, Ireland, operates globally and has a significant presence across key regions.
In North America, the company dominates with extensive activities in the U.S. and Canada. In Europe, the Middle East, and Africa (EMEA), Medtronic is active in both Western and Eastern Europe, as well as in the Middle East and Africa, focusing on expanding healthcare access.
In the Asia-Pacific region, the company is growing rapidly in China, India, Japan, and Southeast Asia. In Latin America, Brazil and Mexico are major markets, complemented by operations in other countries like Argentina and Chile.
This widespread geographical reach supports Medtronic’s mission to enhance global healthcare through its diverse portfolio of medical technologies and services.
Recent Developments
- In September 2024, Medtronic opened a Robotics Experience Studio in Southeast Asia.
- In December 2023, Medtronic’s PulseSelect PFA System was approved by the FDA for treating paroxysmal and persistent atrial fibrillation.
Sun-Pharmaceutical
Company Overview
| Establishment Year | 1983 |
| Headquarter | Mumbai, India |
| Key Management | Dilip Shanghvi (MD) |
| Revenue (US$ Bn) | $ 5.8 Billion (2024) |
| Headcount | ~ 41,000 (2023) |
| Website | https://sunpharma.com/ |
About Sun Pharma
Sun Pharma has been proactive in the mental health treatment providers/sector, focusing on expanding its portfolio of treatments.
Recently, they completed the acquisition of Concert Pharmaceuticals, broadening their range of innovative therapeutic options with the addition of a new drug, deuruxolitinib, now approved for treating severe alopecia areata, which might also impact mental health due to its psychological effects on individuals.
This acquisition aligns with Sun Pharma’s strategic emphasis on enhancing its offerings in specialty segments, particularly those with unmet medical needs.
Moreover, the company’s consistent engagement in rigorous clinical research underlines its commitment to advancing medical science across various therapeutic areas, including mental health.
Geographical Presence
Sun Pharmaceutical Industries Ltd., headquartered in India, is a leading global pharmaceutical company with a substantial presence across several key regions.
In India, it is the largest pharmaceutical firm by market capitalization, with numerous manufacturing and R&D facilities. In the U.S., Sun Pharma ranks among the top pharmaceutical companies and operates multiple manufacturing sites.
Its European footprint has expanded through acquisitions, while in Latin America, the company has grown its market share through direct entry and partnerships.
In the Asia-Pacific region, including China, Japan, Australia, Africa, and the Middle East, Sun Pharma is enhancing its presence through local operations and strategic initiatives, demonstrating its commitment to global market expansion.
Recent Developments
- In August 2024, Sun Pharma agreed to invest US$15 million in Pharmazz.
- In July 2024, Sun Pharma received FDA approval for its oral JAK inhibitor, Leqselvi (deuruxolitinib), for the treatment of severe alopecia areata.
Pfizer
Company Overview
| Establishment Year | 1849 |
| Headquarter | New York City, U.S. |
| Key Management | Albert Bourla (CEO) |
| Revenue (US$ Bn) | $ 58.5 Billion (2023) |
| Headcount | ~ 88,000 (2023) |
| Website | http://pfizer.com/ |
About Pfizer
Pfizer has been actively enhancing its portfolio in the mental health treatment providers/sector, demonstrating a strong commitment to improving mental health care.
The company recently made headlines by offering free public access to mental health assessment tools like the Patient Health Questionnaire (PHQ) and the General Anxiety Disorder-7 (GAD-7), which are vital for diagnosing and assessing mental health conditions.
This move aims to support healthcare providers and enhance patient care by facilitating better diagnosis and ongoing patient assessment.
Moreover, Pfizer has been integrating these initiatives with their broader health strategy. Further, For example, they acquired Seagen for $43 billion, primarily to expand their oncology portfolio but also to support their overall healthcare innovation strategy, which indirectly benefits all therapeutic areas, including mental health.
Additionally, Pfizer’s annual review highlighted their continued focus on innovation and expansion in therapeutic solutions, which underscores their commitment to advancing healthcare, including mental health.
Geographical Presence
Pfizer Inc. boasts a comprehensive global presence with significant operations across North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa.
Headquartered in New York City, Pfizer’s U.S. operations include extensive research, development, and manufacturing facilities.
In Europe, the company has a notable footprint in Western markets such as the UK, Germany, France, and Italy, as well as growing activities in Eastern Europe.
Asia-Pacific is a key region for Pfizer, with substantial investments in China, India, and Japan. In Latin America, Pfizer is active in Brazil and Argentina, while in the Middle East and Africa, its operations include major activities in South Africa and the UAE.
This global network supports Pfizer’s strategic goals, allowing it to address diverse healthcare needs and drive innovation worldwide.
Recent Development
- In August 2024, Pfizer entered a strategic partnership with Flagship Pioneering and Quotient Therapeutics to identify new therapeutic targets for cardiovascular and renal diseases.
- In August 2024, Pfizer invested AU$150 million to enhance its pharmaceutical production facilities in Australia.
Teva-Pharmaceutical
Company Overview
| Establishment Year | 1901 |
| Headquarter | Tel Aviv, Israel |
| Key Management | Richard Francis (CEO) |
| Revenue (US$ Bn) | $ 15.8 Billion (2023) |
| Headcount | ~ 37,851 (2023) |
| Website | http://www.tevapharm.com/ |
About Teva Pharmaceuticals
In 2023, Teva Pharmaceuticals has advanced its mental health treatment efforts with notable innovations. The FDA approved UZEDY, a long-acting injectable risperidone for schizophrenia that helps delay relapse.
Teva also partnered with Medincell on TEV- ‘749, a promising once-monthly injectable olanzapine for schizophrenia, which performed well in phase 3 trials.
Additionally, Teva has expanded its “Community Routes: Access to Mental Health Care” program, donating over $17 million in medications to uninsured patients across the U.S., benefiting over 650,000 individuals with conditions such as anxiety and depression.
This initiative underscores Teva’s commitment to improving mental healthcare access and addressing healthcare disparities.
Geographical Presence
Teva Pharmaceuticals has a robust global presence, with significant operations across key regions. In North America, the company has a major foothold in the U.S. and Canada, focusing on both generics and specialty medications.
In Europe, Teva operates extensively in Western Europe (Germany, France, Italy, Spain, and the UK) and Eastern Europe (Poland, Russia, and Hungary).
Latin America sees Teva active in Brazil, Argentina, and Mexico. The Asia-Pacific region includes operations in India, China, Australia, and New Zealand.
In the Middle East and Africa, Teva is established in South Africa and is working to expand its reach across other African nations.
This broad geographical footprint supports Teva’s strategy of addressing diverse market needs and leveraging regional opportunities globally.
Recent Developments
- In June 2024, Teva Pharmaceuticals introduced an authorized generic of Victoza (liraglutide injection 1.8mg) in the US.
- In May 2024, Teva Pharmaceuticals released results from the efficacy phase of the Phase 3 SOLARIS trial, which assessed TEV- ‘749—a subcutaneous olanzapine injection—in adult patients with schizophrenia, showing it compared favorably to a placebo.
AbbVie
Company Overview
| Establishment Year | 2012 |
| Headquarter | North Chicago, Illinois, United States |
| Key Management | Richard A. Gonzalez (CEO) |
| Revenue (US$ Bn) | $ 50.3 Billion (2023) |
| Headcount | ~ 50,000 (2023) |
| Website | http://abbvie.com/ |
About AbbVie
AbbVie Inc. is advancing in mental health treatment through several strategic initiatives. The FDA recently approved Vraylar® (cariprazine) for major depressive disorder, broadening its use.
AbbVie also strengthened its neuroscience pipeline by acquiring Cerevel Therapeutics, which adds promising psychiatric and neurological treatments.
Additionally, a new collaboration with Gilgamesh Pharmaceuticals aims to develop innovative neuroplastogens for mood and anxiety disorders, seeking to reduce the side effects of existing therapies.
These efforts highlight AbbVie’s dedication to improving mental health care and expanding treatment options.
Geographical Presence
AbbVie Inc. operates globally and has a significant presence across multiple continents. In North America, the company is headquartered in North Chicago, Illinois, and has strong operations in the U.S. and Canada.
In Europe, AbbVie is prominent in the UK, Germany, France, Italy, and Spain. The company has a notable footprint in the Asia-Pacific region, including Japan, China, Australia, and New Zealand. In Latin America, AbbVie is active in Brazil, Mexico, and other countries.
Additionally, in the Middle East, AbbVie is expanding its presence in the UAE, Saudi Arabia, and other regional markets. This broad geographical reach supports AbbVie’s strategic focus on key markets and regional needs.
Recent Developments
- In May 2024, AbbVie and Gilgamesh Pharmaceuticals collaborated to develop advanced therapies for psychiatric disorders, merging AbbVie’s psychiatry expertise with Gilgamesh’s research platform to discover novel neuroplastogens.
- In December 2023, AbbVie’s acquisition of Cerevel Therapeutics boosted its neuroscience pipeline with promising candidates for conditions like schizophrenia, Parkinson’s disease, and mood disorders, adding transformative assets to address significant unmet needs in psychiatric and neurological care.
Discuss Your Needs With Our Analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)




