LONDON, April 21, 2022 /PRNewswire/ —Icometrix the worldwide leader in digital health technology solutions for neurological conditions, announced the recent release of a Medtech Innovation Briefing (MIB) [MIB291] from the internationally renowned NICE for the CE markers and FDA cleared icobrain MRI measures in Multiple Sclerosis (MS).
icobrain can enhance standard of care for people with MS through AI-supported MRI analysis & data-driven care management
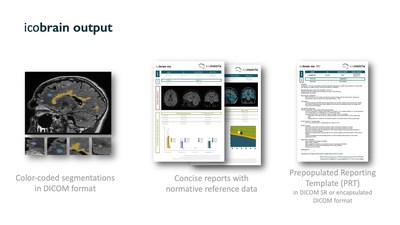
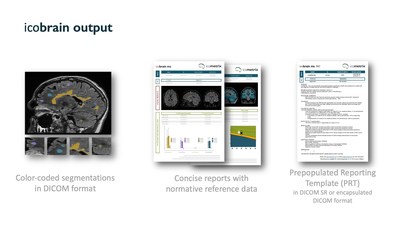
In Multiple Sclerosis, software guided MRI measures of new and enlarging lesions and brain shrinkage are rapidly becoming the standard of care in MS clinical and therapeutic decision-making. The icobrain deep learning AI solution, developed by icometrix, is the most widely adopted and best validated clinical tool for individual people with MS. The added value of icobrain compared to current practice has been acknowledged by experts in the field, leading to the first MIB for Multiple Sclerosis care being issued.
“In MS, it is crucial to detect disease activity early for best informed treatment decisions and outcomes”, states Prof. Klaus Schmierer from Queen Mary University of London and Barts Health NHS Trust. “In addition to clinical scores, neurologists rely heavily on MRI to make therapeutic decisions. Hence, there is a significant need for sensitive and accurate MRI indices”, he continues. “This NICE MIB is an acknowledgement of icobrain as a leading competitor in the field of AI-supported MRI analysis and, more generally, data-driven care management with potential to enhance the standard of care for people with MS in the UK and beyond”, Prof. Schmierer concludes.
“We are thrilled by this MIB recognition from NICE and the experts in this field”, Dr. Wim Van Hecke, founder and CEO of icometrix, comments. “We are proud to lead digital health innovation in the field of MS (and neurological conditions in general), with this first MIB in the field of MS. Being spun out of academics, science and validation are in our DNA. We all know that digital health technology is a crucial component of personalized medicine in neurology, but the importance of clinical validation cannot be underestimated”, Dr. Van Hecke concludes.
Read the NICE Medtech Innovation Briefing [MIB291] here
About icometrix
Founded in 2011, icometrix (Leuven, Belgium; Boston, USA) strives for data-driven insights and personalized patient care, supported by artificial intelligence. icometrix offers a portfolio of eight regulatory approved AI solutions to assist healthcare with various challenges; icobrain extracts data from brain MRI and CT scans for the radiological reporting and clinical management of neurological disorders such as multiple sclerosis, brain trauma, epilepsy, stroke, dementia, and Alzheimer’s disease. icompanion, a digital platform, and mobile app helps people with MS and their care team to monitor clinical symptoms and treatments efficiently and objectively. icolung was one of the first available AI solutions to support clinicians responding to the rapidly evolving needs of the COVID-19 pandemic.
Today, icometrix is internationally active and integrated into more than 100 clinical practices. In addition, icometrix supports pharmaceutical companies in phase I-III and Real-World Evidence (RWE) studies through imaging and data services, and digital health strategy.
Contact info:
Info@icometrix.com
https://www.icometrix.com
BE: +32 16 369 000
US: +1 617 528 0980
Kolonel Begaultlaan 1b / 12
3012 Leuven
Belgium
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/icometrixs-mri-measures-for-people-with-multiple-sclerosis-receive-medtech-innovation-briefing-from-the-national-institute-for-health-and-care-evidence-nice-301530096.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/icometrixs-mri-measures-for-people-with-multiple-sclerosis-receive-medtech-innovation-briefing-from-the-national-institute-for-health-and-care-evidence-nice-301530096.html
SOURCE icometrix