Introduction
Gastrointestinal parasitic infections are one of the global health concerns in developing countries like Bangladesh. Among them, Cryptosporidium spp. plays an essential role in causing diarrhea, malnutrition, and poor cognitive function, especially in children. This study was conducted to identify the frequency of Cryptosporidium cases and other parasitic agents.
Methods
A cross-sectional observational study was conducted among 219 hospitalized children with diarrhea. The conventional microscopic technique was applied for parasitic detection. Particular staining (modified Ziehl-Neelsen) procedure was performed to identify oocysts of Cryptosporidium spp. A polymerase chain reaction (PCR) was performed to determine the SSU rRNA and gp60 gene of Cryptosporidium.
Results
Cysts of Giardia duodenalis (2.3%), ova of Ascaris lumbricoides (1.4%,), Trichuris trichiura (0.5%), and both A. lumbricoides and T. trichiura (0.9%) were identified in samples through wet mount preparation. The distribution of Cryptosporidium spp. as detected by the staining method and nested PCR was 1.4% and 4.1%, respectively.
Conclusion
Factors independently associated with Cryptosporidium infection are unsafe water, lack of regular hand washing, and insufficiency of exclusive breastfeeding. This study reports, presumably for the first time, the detection of Cryptosporidium oocysts in Chattogram metropolitan city of Bangladesh.
Introduction
Diarrhea is the passage of three or more liquid or loose stools per day or if the individual experiences more frequent passage than usual [1-3]. It is categorized clinically as i) acute watery diarrhea, which persists for several hours or days [4], ii) persistent diarrhea that lasts for 14 days or longer [5], and finally, iii) dysentery, blood, and mucous found in diarrheal stool [6,7]. Previously, diarrhea was one of the deadliest diseases [8,9] and remains the second dominant cause of child mortality worldwide [10,11]. Almost 1.7 billion cases of childhood diarrheal diseases are diagnosed each year globally, resulting in the annual death of approximately 525,000 children under five, around 63% of the reported global diarrhea burden [12]. Consequently, acute diarrheal disease remains the top cause of morbidity and mortality among the pediatric population after a respiratory illness, especially in low-middle-income countries, and creates a serious public health issue [13,14]. It is liable for one in eight deaths among children less than five years in Africa, Asia, and South America per annum, or roughly 499,000 children are incriminated of diarrheal disease [13]. The vast majority occurs in Sub-Saharan Africa, reflecting this region’s highest child death rate [14-16].
A single episode of moderate-to-severe diarrhea has significant repercussions on mortality and linear growth among survivors, facilitating the hazards of growth retardation, ill health, and cognitive impairment in the pediatric community [17-19]. Diarrheal diseases are a significant public health problem that affects children in developing countries where insufficient sanitation, hygiene, and portable water supply are the critical factors [13,20-25]. In Bangladesh, one-third of the child death burden is due to diarrhea, a life-threatening disease [26-28]. It was observed that a rural child suffers from 4.6 episodes of diarrhea and about 230,000 children die among them per year [26]. Therefore, it is essential to identify the etiology with the proper diagnostic procedures, followed by disease management interventions are then possible.
Etiological agents of diarrhea
The rotavirus is the most prevalent among numerous viral, bacterial, and parasitic agents causing pediatric diarrhea [29,30]. Some bacterial enteropathogens are also responsible for generating the same, such as Shigella, nontyphoidal Salmonella (NTS), Diarrhoeagenic Escherichia coli (DEC), Vibrio cholerae, Campylobacter, and Yersinia spp. [23,31,32]. Although enteric viruses and bacteria remain the predominant etiological agents [33-35], intestinal protozoal parasites also add related helminths that are also significantly related to diarrheal disease in children, including Cryptosporidium spp., Giardia duodenalis, Entamoeba histolytica, Blastocystis hominis, Dientamoeba fragilis, Ascaris lumbricoides, Trichuris trichiura, Ancylostoma duodenale, and Necator americanus [36-38].
Cryptosporidium as a protozoan diarrheal agent
An intracellular apicomplexan protozoan parasite belonging to the genus Cryptosporidium is second only to rotavirus as the leading cause of moderate-to-severe diarrhea [39-41]. The organism attracts significance as one of the most prominent causes of diarrhea and diarrhea-causing death in young children, especially among infants and immunodeficient individuals in developing countries worldwide [40,42,43]. As the annual detection rate of Cryptosporidium-ascribable cases is about 2.9-4.7 million in children under two years, the sub-Saharan African and South Asian territories face tremendous challenges in combating this infection [44].
Various species of this parasite have been identified and distinguished regarding host range and public health concerns [43]. Thus, Cryptosporidium, the ubiquitous coccidian parasite, has over 40 established species, and of these, 20 species and subtypes account for the vast majority of human gastrointestinal infections worldwide. Additionally, C. hominis and C. parvum are the leading culprits causing cryptosporidiosis globally [45, 46]. Transmission occurs with fecal-oral transmission, either by zoonotic or anthroponotic transmission. Cryptosporidium spp. has a low infective threshold with a robust oocyst that survives adequately in moist, ambient environments and is resistant to many commonly available disinfectants such as chlorine [18,47].
Effects of Cryptosporidiosis
Diarrhea associated with cryptosporidiosis has been linked to three fundamental mechanisms [40,48,49]: i) osmotic diarrhea caused by malabsorption [50,51]; ii) parasite-induced production of inflammatory products and host neurohumoral secretagogues [48,49], and iii) secretory diarrhea caused by a parasite enterotoxin [52-54]. Different absorptive and secretory characteristics exist in different parts of the gastrointestinal system [55]. In immunocompetent persons, the small intestine, principally the ileum, is the primary location of Cryptosporidium infection. However, in AIDS patients, the gastrointestinal parasite distribution is more complex and extensive [56,57].
The infection ranges from asymptomatic, self-limiting diarrhea to chronic [58-60]. The disease’s intensity depends on the individual host’s age, nutrition, and immune status [18]. The disease disrupts the intestinal epithelium, impairs the absorptive and barrier function of the small intestine, and initiates prolonged (7-14 days) and persistent (≥14 days) diarrhea [18,61]. Though cryptosporidiosis is a self-limiting disease in immunocompetent individuals, the aftermath of this illness has far-reaching threatening hazards beyond diarrheal consequences. It also interferes with nutrient absorption, resulting in chronic malnutrition, poor growth, and premature mortality, especially in developing countries [37,62]. Despite enlisting Cryptosporidium in its “Neglected Disease Initiative 2004” by WHO, it’s one of the significant causes of diarrhea in children [63]. Several factors are related to this infection, such as host, environmental, and parasite species. Long-term contact with domestic animals, overcrowded places, poverty, poor sanitation, contaminated water sources, the immune status of the individual, and malnourishment in children also play a crucial role in this infection [40]. Recent studies highlight a reduction in overall diarrheal episodes in Bangladesh by improving water sources and sanitation behavior. Still, cryptosporidiosis and growth faltering, a recognized upshot of this infection, haven’t decreased [64,65].
Studies on Cryptosporidium
Epidemiologic studies have demonstrated that Cryptosporidium is more prevalent in developing countries than in developed ones [25,40,42,66]. The organism has been reported as a substantial burden causing acute diarrhea [67]. A meta-analysis study on consequences of childhood diarrhea caused by this protozoan infection showed that, in 2016, it was the fifth most significant diarrheal etiological pathogen globally in children younger than five years, and acute infection attributed to more than 48,000 deaths and more than 4·2 million disability-adjusted life-years lost [18]. The significance of this intestinal parasite can easily be realized by the current Global Enteric Multicentric Study (GEMS) done on children from seven Asian and African countries, where 9,439 were moderate-to-severe diarrheal cases and 13,129 control subjects, unveiling four exclusive moderate-to-severe diarrhea-causing agents namely Rotavirus, Cryptosporidium, enterotoxigenic E. coli, and Shigella [68].
In a longitudinal cohort study on 392 Bangladeshi slum-dwelling children (in the first two years of life) performed from 2008 to 2014, Cryptosporidium infection was widespread (77%). The study also highlights a close correlation between poverty and stunted growth during the first two years of life [40]. A survey of over 423 fecal samples from 185 children (up to five years) in an urban slum area of Bangladesh was done. Cryptosporidium oocyst was detected in 9.2% of cases, where the infection was highest among children aged less than two. Moreover, that study also observed the infection decreases with age [69]. In a prospective study, fecal samples from children under 16 years attending an outpatient clinic in Cambodia were examined for Cryptosporidium, where these protozoan oocysts were detected in 2.2-7.7% of cases [61]. In Tehran, a study was done in which stool samples from children below 12 years with diarrhea were collected, and 1.1% of these cases were found positive for this infection [66]. Another Iranian study reported that Cryptosporidium oocysts were detected in 3.8-8% of pediatric and immunocompromised patients [68].
Diagnostic modalities
The diagnostic procedure comprises the microscopic examination of the fecal sample by wet mount and staining by modified Ziehl-Neelsen (mZN) staining [68] or auramine-phenol staining technique [70]. Direct and indirect immunofluorescence assays are expensive, but oocysts are readily identified [48,71]. An immunological method (enzyme immunoassay (EIA), enzyme-linked immunoassay (ELISA), and immunochromatographic technique (ICT)) provides good sensitivity over microscopy but has drawbacks, including false-positive results, and is unavailable in developing countries due to cost ineffectiveness [72-74]. Though commonly used and cost-effective, the microscopic procedure is labor-incentive and time-consuming, and the process exclusively relies on an individual’s skill and experience [57,75]. Moreover, molecular methods are extensively used for genotyping and molecular epidemiological studies because of their higher sensitivity (detection ranges from 1-106 oocysts) over the traditional microscopic and immunological procedure [45,76,77].
Various genes of Cryptosporidium are documented targeting its species [78], such as small subunit rRNA (SSU rRNA), Cryptosporidium outer wall protein (COWP), 70-kDa heat shock protein (HSP 70), thrombospondin-related adhesive protein (TRAP-C2), dihydrofolate reductase (DHFR), and actin genes [45,79-81]. SSU rRNA and gp60 are the most common genetic markers for Cryptosporidium species identification and subtype determination [82-84]. The SSU rRNA gene is considered extensively used in genotypic differentiation between infections belonging to both humans and animals. In addition, the 60-kDa glycoprotein (gp60) gene possesses highly variable regions that permit many intraspecies sequence heterogeneity. These sites are crucial to determining many C. parvum and C. hominis subspecies [45,57]. The majority of the pathogenic strains are detected by nested assay, which is also chosen for identifying the negligible amount of oocyst (<100) in the specimen [57]. As the parasite has a long-term detrimental effect on childhood growth, nutrition, and cognitive function with no appropriate drug or vaccine strategy, a study on this parasite carries much importance. To date, no such research was previously done in Chattogram city. Hence, this study was designed to detect this pathogen and other parasites as a parasitic etiological cause of diarrhea in pediatric patients in this metropolitan city.
In the current study, microscopy by wet mount preparation, concentration technique (formalin-ether sedimentation technique), and staining procedure (mZN) were applied to all samples. Subsequently, nested PCR was performed targeting SSU rRNA and the gp60 gene. The study would help the clinician diagnose the case properly by exploring the actual etiology of protozoa-related diarrheal illness and indirectly minimizing the empirical use of antibiotics to treat parasitic diarrhea, thus reducing the multi-drug resistant problem in Bangladesh.
Objectives of the study
The objectives of the study include i) identification of intestinal parasites microscopically by wet mount preparation, ii) identification of Cryptosporidium microscopically by mZN staining technique, iii) detection of the presence of Cryptosporidium spp. by nested PCR, and iv) comparison of the result of microscopy with that of PCR.
This paper was previously posted to the Preprints preprint server on March 7, 2022 (https://www.preprints.org/manuscript/202203.0108/v1).
Materials & Methods
Study details
This was a cross-sectional observational study conducted in the Department of Microbiology, Chittagong Medical College, Chattogram, Bangladesh, and Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University (CVASU), Chattogram, Bangladesh, from July 2019 to June 2020. The study population included indoor and outdoor pediatric diarrheal patients of Chittagong Medical College Hospital and Chattogram Maa-O-Shishu Hospital Medical College, Chattogram, Bangladesh.
The study received ethical approval from the Institutional Review Board of Chittagong Medical College Hospital (CMCH), Chattogram, Bangladesh (Approval number CMC/PG/2019/592, dated November 3, 2019). The research method was strictly aligned with the Declaration of Helsinki. Participation and reporting were hinged on consent forms signed by patients/guardians of the pediatric diarrheic patients (children up to 18 years) before administering the questionnaire. The respondents were informed correctly using the participant’s information sheet about their rights and all the relevant aspects of the study, including its aim and interview procedure. No patient was older than 18 years.
Sample size calculation and technique
n= z2pq/d2==123.2. The n is the sample size. z is the confidence interval (95%) z=1.96. p is the pre-estimated prevalence of 9.2% obtained from a study performed by Ahmed et al. conducted in Dhaka, Bangladesh [85]. d is a marginal error (5%). n = (1.96)2 x0.09 2×0.908/ (0.05) 2=123.17. This means a minimum of 123 participants need to participate in this study.The sampling technique was a nonprobability, purposive type of sampling.
Eligibility criteria
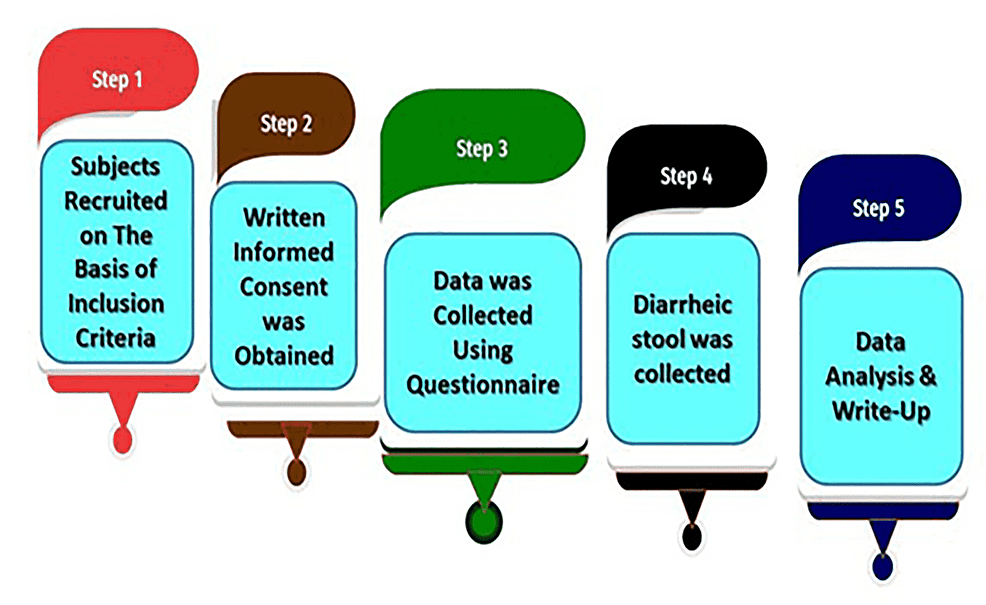
This study was conducted on both inpatients and outpatients. Bangladeshi pediatric patients (up to 18 years) suffering from acute watery and persistent diarrhea (≥14 days) irrespective of their social status [27,86,87], who agreed or whose guardians gave consent to enroll their children after the complete explanation of the study objectives were included.Pediatric patients having bloody diarrhea (with blood and mucous) and above the age of 18 years were excluded. Moreover, patients or guardians who were unwilling to sign the assent form were excluded. The macroscopic study plan is illustrated in Figure 1.
Figure 1: Flowchart depicting the macroscopic study plan
Preparation of questionnaire
A questionnaire was prepared and modified from the study conducted by Tombang et al. in Cameroon [88]. The questionnaire was adjusted using the study’s eligibility criteria and Bangladeshi cultural aspects. In the first section of the questionnaire, particulars of the patients were included. This was followed by socio-demographic history, habitual elements, and clinical features.
Sample collection
After taking consent, the patient’s history details, including demographic information and clinical findings, were recorded in a predesigned case record form. Then stool sample was collected in a clean, leak-proof, wide-mouth container appropriately labeled with the patient’s name, age, time of collection, and identification number. It was then transported to the Department of Microbiology, and some portions of each sample were transferred to the Eppendorf tube and refrigerated at -80ºC to perform the molecular method [89]. The remaining sample was transferred into two containers. One container contained a 10% formalin preserved fecal sample for direct wet mount preparation, and the other retained an unpreserved fecal sample. This was followed by the concentration procedure which consisted of the following step: i) By using a stick, an estimated 1gm of feces was emulisified in about 4 ml of 10% formol water contained in a tube, ii) A further 3-4 ml of 10% v/v formol water was added, the tube was capped, and mixed well by shaking, iii) The emulsified feces were sieved collecting the sieved suspension in a beaker, iv) the solution was transferred to a centrifuge tube and 3-4 ml of ethyl acetate was added, v) The tube was then vortexed for 15 minutes, vi) It was centrifuged immediately at low speed 1000 rpm for one minute, vii) A pipette was used to remove the entire column of fluid below the fecal debris and ether and this was transferred to another centrifuge tube, viii) Formol water was added to make the volume up to 10 to 15 ml and centrifuged at 3000 rpm for 5-10 minutes, ix) The supernatant was removed and the bottom of the tube was tapped to resuspend and mix the sediment [90-92] was followed with this unpreserved sample before the staining procedure. The sediment was now ready to make a smear on the slide for microscopic examination.
Laboratory procedure
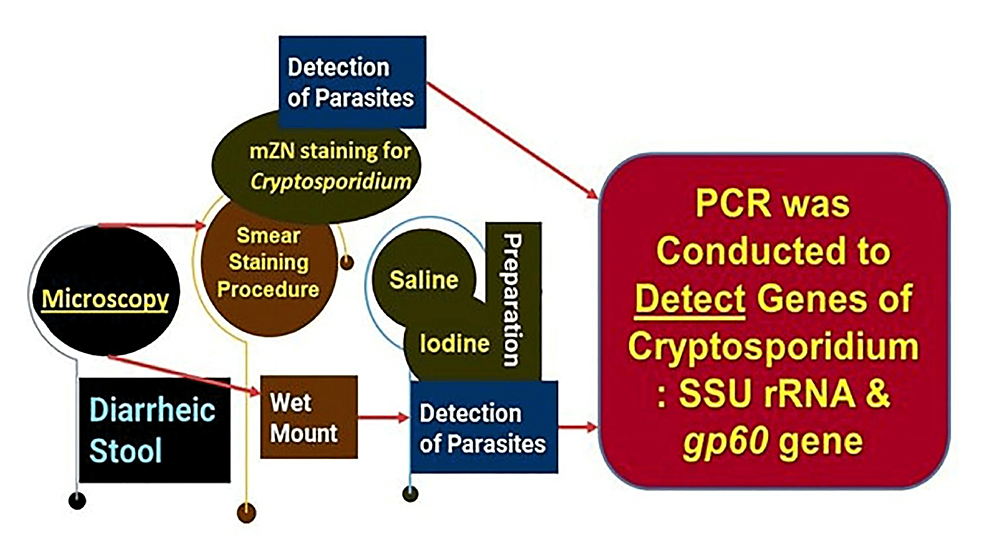
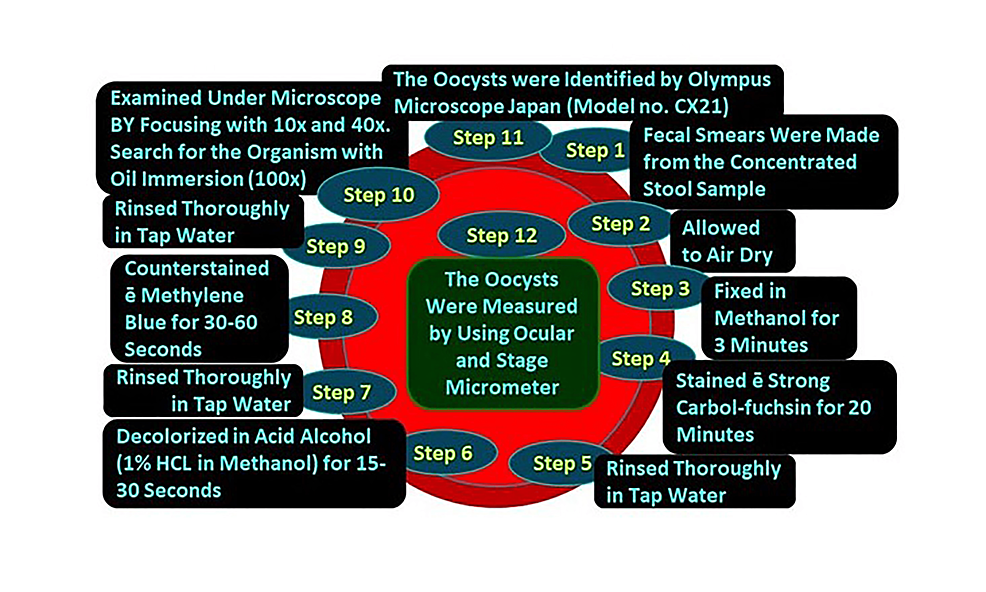
All concentrated samples were subjected to wet mount preparation by both saline and iodine preparation (saline wet mounts and iodine wet mount were arranged by discreetly blending a small volume of stool sample with a drop of physiological saline, methylene blue dye, and Lugol’s iodine (diluted in 1: 5 distilled water), respectively, on a glass slide and employing a coverslip over the smear) [93,94] and staining by mZN (Figure 2 and Figure 3). A fecal smear was made from a concentrated stool sample on a clean, grease-free glass slide. After fixation, mZN staining was performed and the slide was examined under a microscope using oil immersion. Cryptosporidium oocysts were identified as red, small (4-6 µm), characteristically round or slightly ovoid, and acid-fast oocysts against a blue background [95].
Figure 2: The steps of laboratory methods employed
PCR: polymerase chain reaction
Figure 3: Steps of modified Ziehl-Neelsen stain
Procedure of PCR
Process of DNA Extraction
DNA extraction was performed according to the manufacturer’s instruction with the Invitrogen PureLink Microbiome DNA Purification kit (Thermo Fisher Scientific, Waltham, Massachusetts, United States) and the procedure was performed at room temperature (20-25°C). The purified DNA in the tube was preserved at -20°C for further use. Primers used for SSU rRNA and gp60Gene [96,97] are given in Table 1. The first pair of primers (Table 1) were used as first-round PCR to amplify the 830 bp sequence of the SSU rRNA gene, and the second pair of primers were used for second-round PCR to amplify a 240 bp sequence. For the gp60 gene, first set primers (Table 1) were used as first-round PCR to amplify the 412 bp sequence, and the next pair of primers were used for second-round PCR to amplify the 350 bp sequence of the gp60 gene.
| Primer name | Primer sequence (5’-3′) | Size | ||
| SSU rRNA gene | First Set Primer | XF2 | F-GGAAGGGTTGTATTTATTAGATAAAG | 830 bp |
| XR2 | R-AAGGAGTAAGGAACAACCTCCA | |||
| Second Set Primer | PSSUf | F-AAAGCTCGTAGTTGGATTTCTGTT | 240 bp | |
| PSSUr | R-ACCTCTGACTGTTAAATACRAATGC | |||
| gp60 gene | First Set Primer | gp15-ATG | F-ATGAGATTGTCGCCTCATTATC | 412 bp |
| gp15-STOP | R-TTACAACACGAATAAGGCTGC | |||
| Second Set Primer | gp15-15A | F-GCCGTTCCACTCAGAGGAAC | 350 bp | |
| gp15-15E | R-CCACATTACAAATGAAGTGCCGC |
Table 1: Primers used for SSU rRNA and gp60 genes of Cryptosporidium species
Preparation of Reaction Mixture
Sterile micro-centrifuge tubes (1.5 ml) were taken and labeled with the date and identification number. Primer tubes were centrifuged for a few seconds. Then it was vortexed for 15 seconds and diluted with nuclease-free water to make a 1:10 dilution. For each sample, a total of 20 µl of the mixture was prepared by mixing 10 µl of master mix (mixture of dNTP, Taq Polymerase, MgCl2, and PCR buffer), 1 µl forward primer, 1 µl of reverse primer, 2 µl DNA template, and 6 µl of nuclease-free water.
Cyclic Condition used for Nested PCR [98]
SSU rRNA Gene of Cryptosporidium Species: In the first round PCR, initial denaturation at 94°C for five minutes was followed by 30 cycles of denaturation at 94°C for 45 seconds, primer annealing at 450C for two minutes, extension at 72°C for 1.5 minutes, and a final extension at 72°C for 10 minutes. In the second round PCR, initial denaturation at 94°C for five minutes was followed by 35 cycles of denaturation at 940C for 30 seconds, primer annealing at 55°C for 30 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 10 minutes. This is shown in Table 2.
| Name of Gene | Steps of PCR | Initial denaturation | Primer annealing | Extension | Final extension |
| SSU | 1st Round PCR | 94°C for 5 minutes followed by 30 cycles of denaturation at 940C for 45 seconds | 45°C for 2 minutes | 72°C for 1.5 minutes | 72°C for 10 minutes |
| 2nd Round PCR | 94°C for 5 minutes followed by 35 cycles of denaturation at 940C for 30 seconds | 55°C for 30 seconds | 72°C for 30 seconds | 72°C for 10 minutes | |
| gp60gene | 1st Round PCR | 94° C for 5 minutes followed by 35 cycles of denaturation at 94°C for 30 seconds | 55°C for 45 seconds | 72°C for 1 minute | 72°C for 10 minutes |
| 2nd Round PCR | 94°C for 5 minutes followed by 30 cycles of denaturation at 94°C for 30 seconds | 55°C for 30 seconds | 72°C for 30 seconds | 72°C for 10 minutes |
Table 2: Cyclic conditions used for nested PCR
PCR: polymerase chain reaction
gp60 Gene of Cryptosporidium Species: In the first round PCR,initial denaturation at 94° C for five minutes was followed by 35 cycles of denaturation at 94° C for 30 seconds, primer annealing at 55°C for 45 seconds, extension at 72°C for one minutes, and a final extension of 72°C for 10 minutes. In the second round PCR, initial denaturation at 94° C for five minutes was followed by 30 cycles of denaturation at 94° C for 30 seconds, primer annealing at 55°C for 30 seconds, extension at 72° C for 30 seconds, and a final extension at 72° C for 10 minutes. This is shown in Table 2.
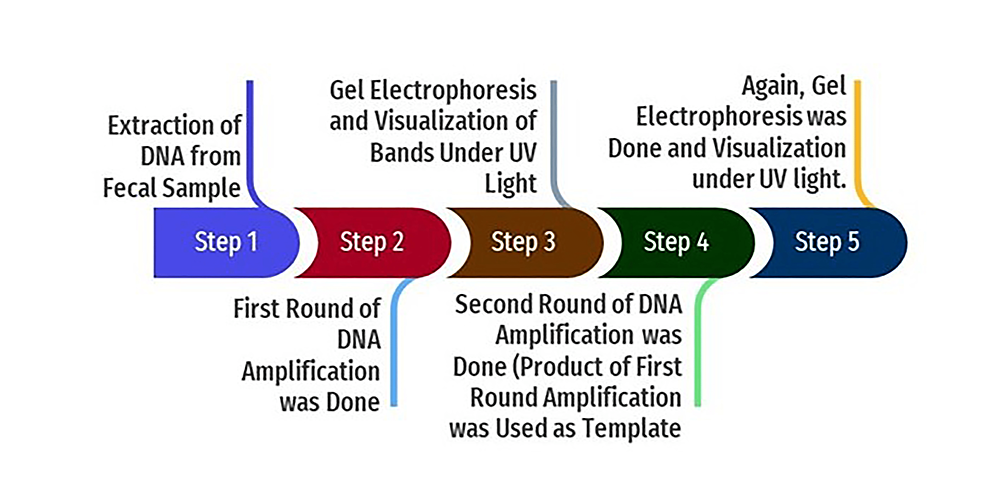
In the nested PCR, the amplified product of the first round of PCR is used as the template. The second set of primers was added to the reaction mixture in the second amplification (nested PCR). The PCR reactions were conducted in a thermal cycler (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts, United States). The amplicon size was determined by comparing the position of the amplicon concerning that of a 100 bp DNA ladder loaded in the adjacent well and simultaneously electrophoresis. Samples were scored as PCR positive for Cryptosporidium spp. when PCR product of 240 bp could be detected for SSU rRNA gene. Samples were detected as PCR positive for Cryptosporidium spp. when 350 bp could be seen for the gp60 gene. The flowchart of nested PCR is given in Figure 4.
Figure 4: Flowchart of nested PCR
PCR: polymerase chain reaction; UV: ultraviolet
Data analysis
A predesigned questionnaire systematically recorded all the relevant information (history), socio-demographic history, clinical findings, and laboratory findings of every case. The data were analyzed using IBM SPSS Statistics for Windows, Version 25.0 (Released 2017; IBM Corp., Armonk, New York, United States) and Stata Statistical Software: Release 15 (2017; StataCorp LLC, College Station, Texas, United States). Statistical analysis was done by standard statistical procedure; Microsoft Excel (Microsoft Corporation, Redmond, Washington, United States) was used to prepare graphs and charts. A p-value <0.05 was considered significant. The multivariate logistic regression model was used to see the association between independent factors and Cryptosporidium infestation. The regression model was adjusted by age and gender.
Results
Distribution of the study population according to their age and sex
A total of 219 fecal samples were collected from pediatric diarrheic patients at two tertiary medical college hospitals. The age range of the patients was two months to 18 years. At first, all samples were examined by direct microscopic examination, then mZN staining, and afterward, nested PCR was done for specific target genes of Cryptosporidium. The majority of the study population belonged to the age group of 1-5 years (47%), followed by the <1 year age group (30.1%) (Table 3). Of them, 125 (57%) were male and 94 (43%) were female. The male to female ratio was about 1.33:1.
| Age Group | Frequency | Infected cases n(%) | Total cases (n) and percentage (%) |
| <1 | 66 | 2 (22.22%) | 30.1% |
| 1-5 | 103 | 7 (77.78%) | 47.0% |
| 6-10 | 37 | 0 | 16.9% |
| 11-15 | 11 | 0 | 5.0% |
| 15-18 | 02 | 0 | 0.9% |
| Total | 219 | 9 | 100% |
Table 3: Distribution of study population by age (n=219)
Microscopic assay
Wet Mount Preparation
All fecal samples were first examined for direct microscopic examination by saline and iodine preparation. Table 4 shows wet mount findings among 219 study samples where Giardia duodenalis was found positive in five (2.3%) samples. Moreover, other parasites like helminthic eggs were found in some samples. Ova of Ascaris lumbricoides, Trichuris trichiura, and mixed infection were detected in three (1.4%), one (0.5%), and two (0.9%), respectively.
| Parasites | Frequency | Percentage (%) |
| Giardia duodenalis | 05 | 2.3 |
| Ascaris lumbricoides | 03 | 1.4 |
| Trichuris trichiura | 01 | 0.5 |
| Ascaris lumbricoides andTrichuris trichiura | 02 | 0.9 |
| No parasite | 208 | 95 |
| Total | 219 | 100 |
Table 4: Detection of parasites by wet mount in the study population (n=219)
Identification of Cryptosporidium by mZN Staining
The microscopic examination of the stool samples through mZN staining showed the presence of Cryptosporidium oocysts (Figure 4) in 3/219 (1.4%) samples (Table 5).
Figure 5: Cryptosporidium oocyst (arrow) in mZN stain
mZN: modified Ziehl-Neelsen
| Microscopic findings | Nested PCR findings | Total | p value | |
| (+ve) | (-ve) | |||
| Modified Ziehl-Neelsen staining | ||||
| Cryptosporidium (+ve) | 03 (33.3%) | 00 (0.0%) | 03 | |
| Cryptosporidium (-ve) | 06 (66.7%) | 210 (100%) | 216 | <0.001 |
| Total | 09 | 210 | 219 |
Table 5: Association of microscopic findings of Cryptosporidium with nested PCR (n=219)
p-value derived from chi-square test. Figures within parentheses indicate the percentage.
Nested PCR findings
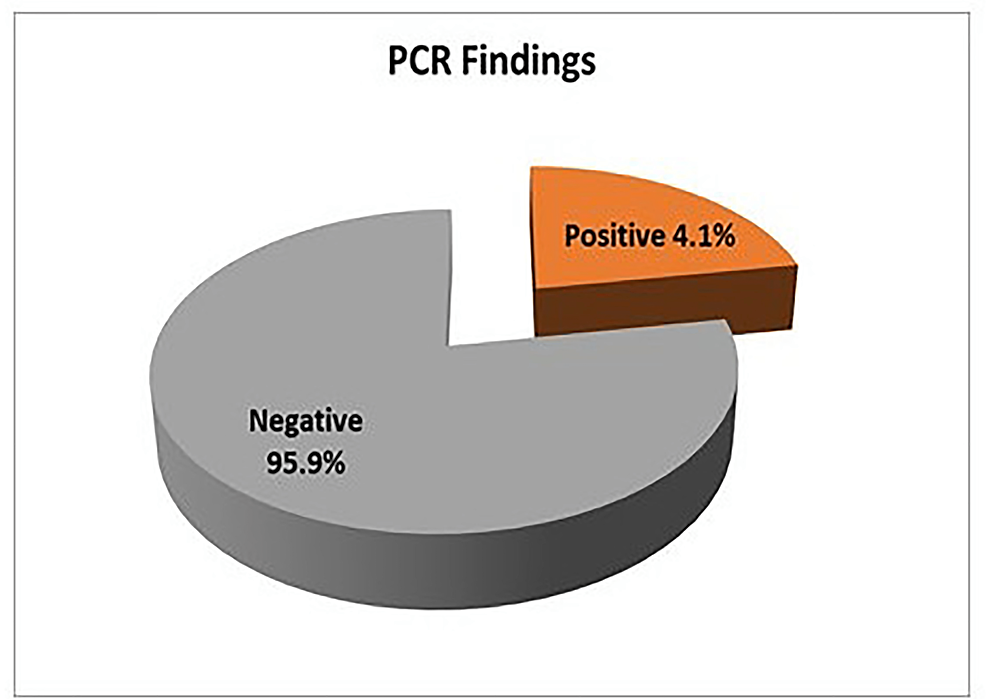
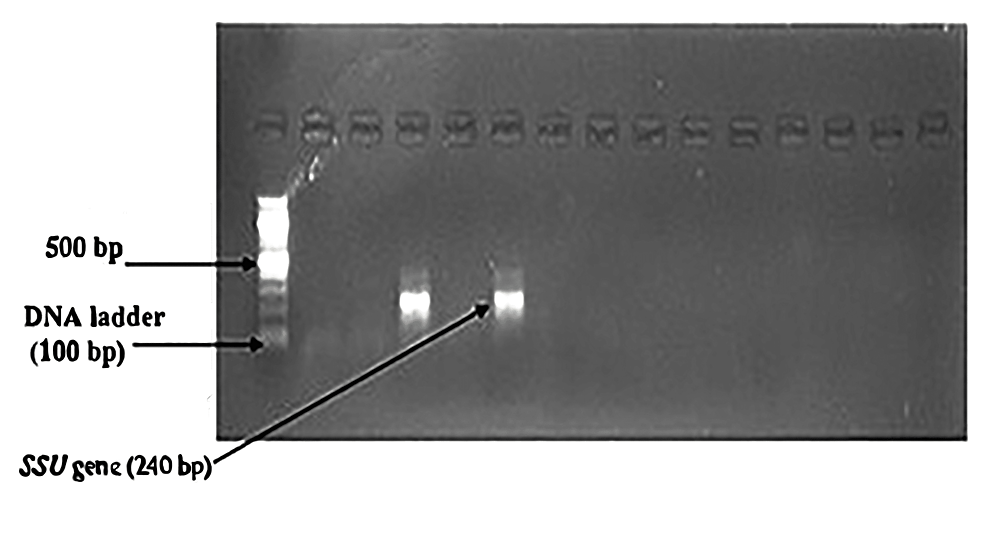
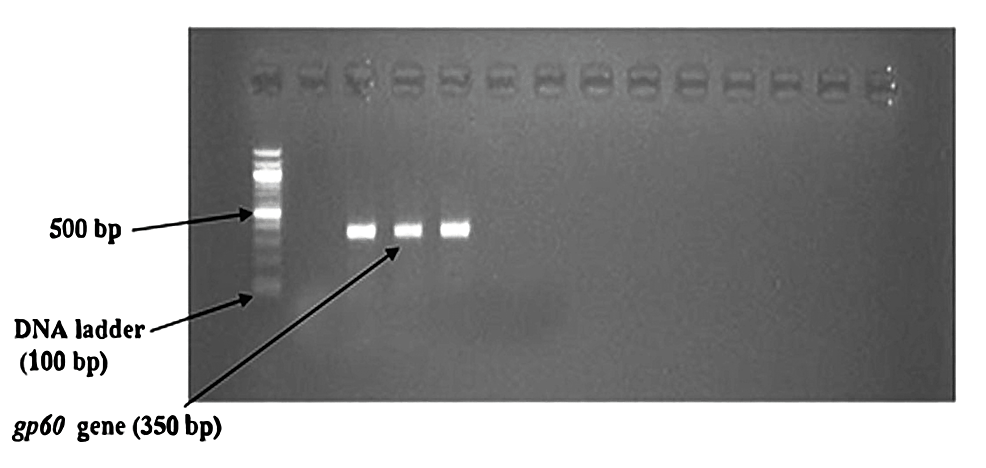
Nested PCR detected Cryptosporidium oocysts in nine (4.1%) samples (Figure 6). The bands of SSU-rRNA (240 bp) and gp60 genes (350 bp) are shown in Figure 7 and Figure 8, respectively.
Figure 6: Distribution of Cryptosporidium spp. by nested PCR (n=219)
PCR: polymerase chain reaction
Figure 7: Bands of amplified DNA of Cryptosporidium spp.
Figure 8: Bands of amplified DNA of Cryptosporidium spp.
The prevalence of Cryptosporidium spp. was more in the 1-5 years age group, and male predominance was observed, but both were statistically non-significant. Table 4 shows the association between microscopic and nested PCR findings of Cryptosporidium. Here the Chi-square test was done, and it was found to be statistically significant (p<0.001). The sensitivity and specificity of Cryptosporidium Spp. microscopy was found to be 33.3% and 100%, respectively, taking the PCR method as the gold standard.
Multivariate logistic regression to determine the independent factors associated with Cryptosporidium infection
Table 6 summarizes the risk factors associated with cryptosporidiosis, including male gender, unsafe drinking water (without boiling or health risk levels of contaminants), irregular hand washing, rural residence, insufficient exclusive breastfeeding, and history of having pets. It reflected the use of unsafe drinking water had a probability of 3.91 times to be infected with Cryptosporidium infestation, OR= 3.91; 95% CI (1.05, 16.1); p=0.049, lack of regular hand washing had a chance of being infected with Cryptosporidium infestation by 9.68 times higher compared to traditional hand washing, OR= 9.68; 95% CI (1.92, 48.4) and insufficiency of exclusive breastfeeding have a risk to infected with Cryptosporidium infestation by 3.82 times higher compared to feed breast milk exclusively, OR=3.82(1.01, 15.3); p=0.049. But other factors like female gender (OR=1.02, P=0.972), rural residence (OR=3.46, P=0.081), and H/O having a pet (OR=0.41, P=0.237) have no significant association with the infection. Most of the study population (64.8%) drink safe water, and 70.3% use regular hand washing.
| Variables | Number of samples for each category | Number of positive Cryptosporidium based on categories | Adjusted OR (95% CI) | P-value |
| Gender | ||||
| Male | 125 (57.1%) | 5 (55.6%) | 1 | |
| Female | 94 (42.9%) | 4 (44.4%) | 1.02 (0.26, 3.94) | 0.972 |
| Drinking water | ||||
| Safe (boiled or appropriate filteratrate) | 142 (64.8%) | 3 (33.3%) | 1 | |
| Unsafe | 77 (35.2%) | 6 (66.7%) | 3.91(1.05, 16.1) | 0.049 |
| Hand Washing | ||||
| Regular | 154 (70.3) | 2 (22.2%) | 1 | |
| Irregular | 65 (29.7) | 7 (77.8%) | 9.68(1.92, 48.4) | 0.006 |
| Residence | ||||
| Urban | 152 (69.4%) | 4 (44.4%) | 1 | |
| Rural | 67 (30.6%) | 5 (55.6%) | 3.46 (0.86, 13.9) | 0.081 |
| Breast feeding | ||||
| Exclusive | 141 (64.4%) | 3 (33.3%) | 1 | |
| Not exclusive | 78 (35.6%) | 6 (66.7%) | 3.82 (1.01, 15.3) | 0.049 |
| H/O having pet | ||||
| No | 178 (81.3%) | 6 (66.7%) | 1 | |
| Yes | 41 (18.7%) | 3 (33.3%) | 0.41 (0.10, 1.79) | 0.237 |
Table 6: Multivariate logistic regression to determine the independent factors associated with Cryptosporidium infection
A multivariate logistic regression model was used to estimate the p-value. The regression model was adjusted by age and sex
CI: confidence interval; OR: odds ratio; s: significant, ns: not significant
Discussion
Intestinal parasites such as Cryptosporidium spp. and other parasites are liable for diarrhea, especially among children in developing countries [99]. Though infections due to these parasites are self-limiting in immunocompetent individuals, chronicity often results in malnutrition, growth faltering, and cognitive function impairment, especially in children [60,100-105]. Because of these alarming effects on a child’s health, it emphasizes the need to establish the incidence of protozoan parasites responsible for childhood diarrheic disease.
In this study, 2.3% cases were found Giardia duodenalis positive by wet mount preparation among 219 samples. The prevalence of Giardia duodenalis infection among 0-15 years Portuguese children was 1.9%, as estimated by direct microscopic examination. Nevertheless, when monoclonal ELISA techniques were utilized, the rate increased to 6.8% [106]. Furthermore, a higher rate (7.8%) of Giardia duodenalis infestation was found in the younger group (0-5 years), and there was no difference observed between the sexes [106]. A study among pediatric patients (below five years) in a tertiary hospital was done where Giardia cysts were found in 4.14% of cases [107]. In a slum area of Bangladesh, Giardia duodenalis positive samples were found in 6.01% of cases among school-going children [108]. In Sikkim, India, a prospective study among symptomatic children (<15 years) was done where Giardia cysts were found in 5% of cases by wet mount [109]. About 8.2% of patients were positive for giardiasis through the direct smear method in Kashmir Valley, India [110]. Another study in Lucknow, India, showed that Giardia was detected in 15.5% by immediate wet mount preparation [111], much higher than the current study findings. This dissimilarity may be due to the large sample size (n=1680) and large age group distribution (3 to 45 years). The geographic area may also be a factor in this difference.
In wet mount film, the current study showed that ova of Ascaris lumbricoides, Trichuris trichiura, and mixed infection were detected in three (1.4%), one (0.5%), and two (0.9%), respectively. A study reported 0.9% Trichuris trichiura [98], but no Ascaris lumbricoides were found in hospitalized pediatric diarrheic children (<5 years), according to our study. Another study demonstrated 0.6% of Trichuris trichiura ova, but Ascaris lumbricoides was 9% [112]. Other studies reported the prevalence of Ascaris lumbricoides and Trichuris trichiura ova as 1.5-8.2% and 0.8-0.9%, respectively [38,113]. In our research, a relatively low number of helminths was detected probably because of the urban setting of the study population, which may have had better sanitation and hygiene practices. Another cause could be the ingestion of anti-helminthic drugs at a regular interval.
Age and sex-specific vulnerability are essential for the prevalence of diarrheal illness, where various factors can contribute to this. These may include biochemical factors like hormones, enzymes, specific proteins, genetic or immunologic factors, food habits, culture, etc. [114]. Cryptosporidium was found positive in 1.4% of diarrheal samples by mZN staining. A surveillance study on children under 12 years old in Tehran was done where the prevalence rate of the protozoa was 1.19% [67]. Another survey on pediatric children (<5 years) reported 2.3% Cryptosporidium oocyst [107]. A study reflected detection rates of Cryptosporidium spp. from outpatients of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR’B) of 4.96% [41]. In Malaysia, the prevalence of these protozoa among diarrheal subjects (<12 years) was 4.62% by mZN staining [115, 116]. In Cameroon, a hospital-based cross-sectional study among children aged <5 years reflects that the prevalence of Cryptosporidium infection was 13.4% in microscopy (mZN staining), having the highest detection rate in the 31-60 months group [88], which is dissimilar to this study. Several factors may play a vital role in this higher prevalence of cryptosporidiosis, such as geographic region, seasonal variation, age, personal hygiene, drinking or using untreated water, no exclusive breastfeeding, low educational status, and poor socioeconomic status of the parents.
In this study, there were some limitations. There was low trophozoite and/or cyst and oocyst detection because of the empirical use of antimicrobials, especially antiprotozoal and anthelminthic. These medications are often self-medicated or given by non-graduate medical practitioners. Additionally, this study could not collect samples from the same patient on multiple occasions. Moreover, microscopic findings depend on individual expertise. Direct microscopic examination after mZN staining of Cryptosporidium relies on morphologic recognition of small-sized oocysts that may be scanty in number, which may be challenging to visualize. Again, they could be inconsistently stained, and misdiagnosis may happen. Therefore, this method is impractical to standardize as it is influenced by the microscopist’s individual skills of the microscopist involved [43]. Moreover, the procedure is incapable of detecting the low parasite count. Collecting at least three stool samples on alternate days is often indicated because of intermittent shedding of protozoan cysts and oocysts. It is worth mentioning that other stages are not visible microscopy. Nevertheless, it’s difficult and inconvenient to go for three samples from the same patient. Though conventional microscopy of more than one fecal sample continues to be recommended to diagnose intestinal protozoa in stool samples, its sensitivity is still low even after multiple examinations. Also, the parasite might be disguised by bile pigment and not visualized by wet mount examination [101]. Since this study was done with a single fecal sample, a low parasite concentration could be missed through microscopy. This could be the reason behind their relatively low rate of detection.
Cryptosporidium spp. was found positive in 4.1% of samples by nested PCR. In one study, the prevalence of Cryptosporidium was 4.8% among children below five years of age with diarrhea [117]. A longitudinal cohort study was done on Bangladeshi slum children where the same procedure found Cryptosporidium positive cases in 6.3% of cases [41]. A prospective study on children under 16 years showed that 7.7% of patients were detected by PCR [60]. Another study found the prevalence of this parasite is about 1.3% [118]. Improved sanitation, safe drinking water, and awareness about health and hygiene enable a lower prevalence of these parasitic diseases in the community [119]. Another cause may lie in examining a single fecal specimen per individual for diagnosis, which is less sensitive than using multiple samples [45,57].
Moreover, the study was performed in urban rather than rural or slum areas. PCR is considered a more sensitive and specific diagnostic method, but some limitations remain such as PCR bands were not confirmed by sequencing because of structural and financial difficulty. There is a list of PCR inhibitors in stool samples, namely lipids, hemoglobin, bilirubin, bile salts, polysaccharides from mucous, bacteria, and food degradation products, which can also affect the result of amplification. Commercial kits, including extraction columns, have been used to purify the DNA to minimize the effect of DNA inhibitors. Despite using such a kit, false-negative results may happen [120,121]. The highest cases of Cryptosporidium were detected in the same age group (one to five years) 7/103 (6.79%), followed by <1-year age group 2/66 (3.03%), which was not statistically significant according to the p-value (p=0.376). Moreover, we revealed from our study that all Cryptosporidium-positive cases were children <3 years old. In another study, it was seen that the highest prevalence of Cryptosporidium infection was in the age group of three to four years (14.3%), followed by <1-year age group (4.5%) [117]. A study found that Cryptosporidium infection was predominant among children <5 years (22%) [50]. Most infected cases were children <4 years of age [117].
Children (1-5 years) develop a habit of putting unwashed hands, toys, and other objects inside their mouths. Moreover, their compromised immunological status and poor hygiene practices are susceptible to intestinal infections, especially during this period [122,123]. The infection rate decreased in higher age groups with minimum infection rate because of the improved immunological status of the individual [40,124]. It is observed that there is a difference in detection rate with other studies. One reason could be that it was a tertiary hospital-based study rather than a rural or slum-based one. On the other side, our study’s low detection rate may suggest an improved living standard for the study participants. It may be due to the improvement of the sanitation and hygiene system. The empirical use of antiparasitic drugs could be one of the reasons behind the low prevalence of intestinal parasites in our country [125,126]. Failure to detect intestinal protozoan parasites may be due to seasonal variation and the intermittent nature of excretion of this parasite in the stool [48,127].
In this study, male predominance was observed in the case of Cryptosporidium Spp. Infection was similar to Hawash et al. [128], but the opposite picture was found in another study where females were affected more [129,130]. This discrimination is unclear because under-five children of both sexes are engaged in the same recreational activity and are likely exposed to the same environmental conditions. Still, it could be because the males constitute most of the study population. The association of microscopic findings with PCR for pathogens showed high statistical significance (p<0.001). The sensitivity of Cryptosporidium microscopy was 33.3%, and specificity was 100% in the present study. Two other studies found similar validity [131,132].
Globally, foodborne cryptosporidiosis has been recognized as the main reason for such infestation [133-135]. Additionally, drinking water from swimming pools, waterparks, fountains, lakes, and rivers contaminated with Cryptosporidium remains one of the common causes of spreading cryptosporidiosis [136-138]. This water source could be contaminated by fecal matter. In some city areas, sewage and toilet wastage may get into the surface water, which could be a potential concern for the fecal-oral transmission of this protozoan parasite. Furthermore, cryptosporidiosis has been positioned fifth among the 24 most significant foodborne parasites [139,140]. The World Bank reported that 98% of Bangladeshi people currently have access to better-quality water. However, water quality remains poor across the country because 80% possess microbial contamination [141]. United Nations Children’s Fund (UNICEF)/WHO reported that about 25% of Bangladeshi people do not have safe drinking water resources in their homes [142]. The results of risk factor analysis support the role of having unsafe drinking water in Cryptosporidium infection that is statistically significant (p-value=0.043), which agrees with another study conducted in Pakistan [143]. Another critical issue is that Cryptosporidium oocysts are highly resistant to numerous disinfectant agents [143-145] and are killed by boiling water above 70° C in less than one minute [146]. In addition, multiple studies, including the WHO guidelines for drinking water quality, suggest that those oocysts become non-viable and inactivated at 60-71.7° C [129,135,143,147,148].
Another salient factor behind protozoal diarrhea is inadequate hygiene practices like handwashing [62,63,149-151]. Our study found a significant association between poor hand washing practice and cryptosporidiosis (p-value=0.001). It may be due to improper washing of hands while handling infant feeding bottles. Moreover, there is a tradition of practicing force-feeding techniques with bare hands to ensure enough food intake by the children to support their growth [152,153]. The hazard of bare hands (not properly washed) enables direct transmission of this foodborne protozoan disease [154,155]. These malpractices encourage ingesting food and water contaminated with oocysts and are shed from infected individuals [156,157].
Undoubtedly, breastfeeding has a widespread impact on children’s health conditions, and there remains a significant correlation between breastfeeding and diarrhea [158-160]. Our study speculated that failure of exclusive breastfeeding was associated with Cryptosporidium infection [130,161]. It can be postulated that the mother’s milk provides numerous antibodies, Immunoglobulin A (IgA), which offers passive protective immunity against various parasitic infections [130,161,162]. Multiple studies reported that parasitic-infested infection is almost prevented till six months of age and when complementary foods are associated with infective diarrhea [117,163,164]. Though contact with animals has been reported as a risk factor for this infection [165-167], we observed a higher distribution of this pathogen in children without animal rearing. Our findings were similar to earlier studies [44,168]. The finding suggests the transmission mode could be the person-to-person contact-no association of the pathogen with the rural resident, supporting some previous studies [118,124,169,170].
Conclusions
The present research can be brought into play that the PCR approach demonstrated better results than the traditional microscopic procedure as it yields more pragmatic outcomes. This procedure also denotes that it can adequately diagnose the parasitic causes of diarrhea among children. Consequently, it brings considerable advantages to the treatment of pediatric diarrheal patients in Bangladesh and other low and middle-income countries (LMICs). As a result, irrational prescribing and self-medication of antimicrobials hopefully be minimized. Thereby, it is expected that the rate of antimicrobial resistance will be cut down. Moreover, this research provides scientific data to detect other protozoal parasitic diarrhea through mentioned methods. Additionally, this study enables information regarding the prevalence of protozoan causes of diarrhea in the locality or region of Bangladesh.
The samples were identified positively by mZN staining for Cryptosporidium spp.; oocyst was successfully proved positive by molecular assay (PCR), which recommends the practice of PCR approaches and conventional microscopic procedures to overthrow the practice the diagnostic drawbacks. So, the staining method should be combined with wet mount preparation as a routine examination for stool to detect and diagnose the parasite effectively. Moreover, molecular analysis is also recommended where facilities are available. This would help to diagnose the protozoa precisely. Further molecular analysis like DNA sequencing can be done, which will provide us with specific information about the species and genotypes, hence, lending the researcher a transparent idea about the epidemiology to identify risk factors, mode of transmission, pathogenicity, and genetic diversity, etc.
Parasites are one of the critical causes of pediatric diarrheic disease in the Bangladeshi community. mZN staining should be introduced as a routine-based detection method for Cryptosporidium spp. mZN is much more cost-effective in considering those countries that have poor budgetary allocation and access to healthcare. Finally, the practice of regular hand washing and consumption of boiled water is an essential factor in minimizing parasitic infection, including cryptosporidiosis