Perspective: Accessing Innovation at the Point of… Leave a comment
Precision oncology has the potential to create a personalized experience for every patient with cancer, but there are still significant disparities in the use of these precision oncology tools between patients from different countries, with low- and middle-income countries often facing inequalities.While the list of actionable biomarkers and US Food and Drug Administration (FDA)-approved molecularly guided therapies grows, comprehensive genomic profiling (CGP) assays, which test for hundreds of genomic biomarkers using next-generation sequencing, will play an important role in improving healthcare efficiency and making precision oncology a reality for all patients. However, this will only occur if there is collaboration between the different stakeholders within the healthcare system — such as industry partners, academia, and regulatory bodies — to increase patient access.
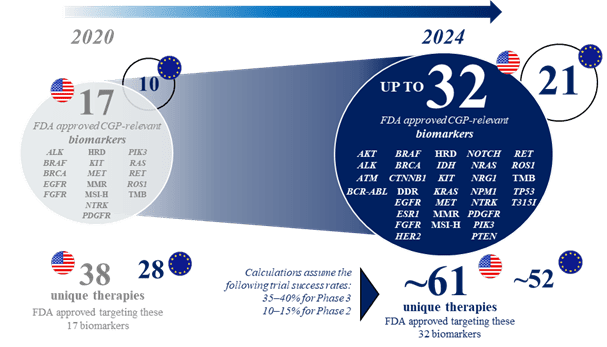
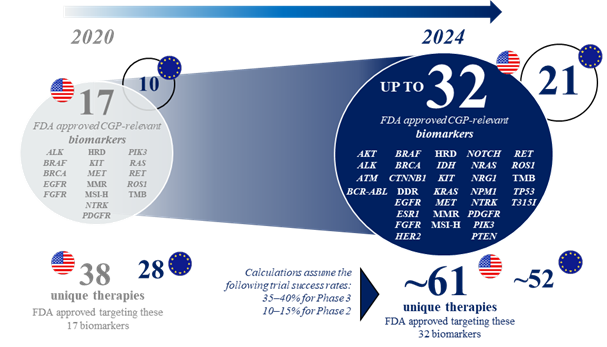
The recent innovation of precision oncology is set to continue, with an increasing number of clinical development programs investigating molecularly guided cancer therapies in recent years. Based on an analysis conducted by Roche of several databases focusing on clinical trials of patients with metastatic cancers, it is predicted that by 2024, there will be 32 genomic biomarkers and 61 corresponding molecularly guided therapies approved by the FDA (21 and 52 for the EU; assuming that the majority of these trials will deliver positive results and regulatory approval is subsequently obtained) (see figure 1). This is a considerable increase from the current 2022 numbers of 17 and 38, respectively (10 and 28 for the EU). The 2024 predictions include 15 approved genomic biomarkers that may be targetable with particular therapies across all cancer types, more than 1.5 times the number approved by 2022 (see figure 2). As an example, in non-small cell lung cancer, the number of genomic biomarkers that may be targetable with particular therapies is expected to be 21 by 2024 (see figure 3).
 Figure 1: Evolving landscape of CGP-relevant biomarkers likely to have approved targeted therapies in solid tumor indications by 2024.*
Figure 1: Evolving landscape of CGP-relevant biomarkers likely to have approved targeted therapies in solid tumor indications by 2024.*
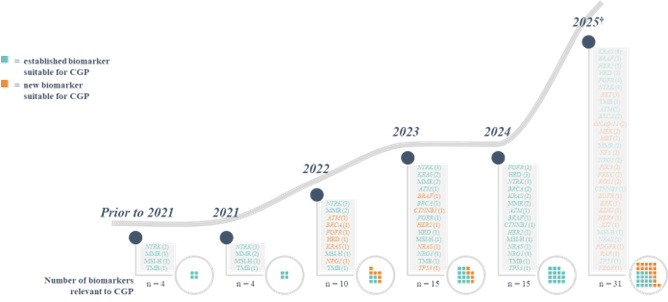 Figure 2: Biomarkers likely to have approved targeted therapies with tumor-agnostic indications.*
Figure 2: Biomarkers likely to have approved targeted therapies with tumor-agnostic indications.*
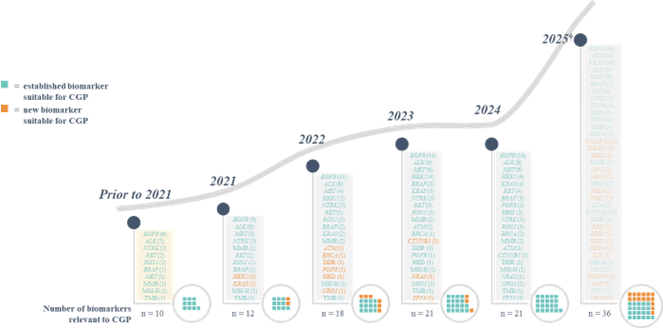 Figure 3: Biomarkers likely to have approved targeted therapies for non-small cell lung cancer.*
Figure 3: Biomarkers likely to have approved targeted therapies for non-small cell lung cancer.*
As the pipeline of molecularly guided therapies grows, using both tissue and liquid-based CGP to select an appropriate therapy for patients becomes more relevant to avoid a waste of resources within the healthcare system. Evidence is emerging that such an approach may offer an increase in efficiency and cost savings within healthcare through reduced time to testing and treatment, and lower resource utilization via fewer hospitalizations and elimination of unnecessary testing and treatment. As a specific example, many patients with metastatic non-small cell lung cancer are automatically deferred to immunotherapy due to a lack of tissue material for evaluation of their genomic alterations. However, broad liquid-based CGP has been shown to offer cost savings versus specific gene testing via increased use of first-line targeted therapy and, concurrently, reduced use of immunotherapy. In addition, as an evolving technology, NGS testing offers the opportunity for earlier cancer detection and diagnosis, which can lead to greater chances of successful treatment, improved patient outcomes and quality of life, and reduced burden of care for the patient, their family, and the healthcare system. However, despite these potential improvements to healthcare efficiency, the increasing number of molecularly guided therapies places an increased strain on payer budgets, as the therapies themselves are the cost drivers in current value assessment frameworks assessing precision oncology.
“Healthcare is the prime example of complexity — yet we tackle it in a unidimensional, often siloed way — this has to change”, said Marlene Thomas head of integrated healthcare solutions at Roche. Evidence-based and collaborative systematic reviews are needed to create fit-for-purpose, harmonized, and transparent value-assessment frameworks that: 1) apply globally, and not just to high-income countries in which health technology assessment and decision-making processes are more mature; and 2) integrate the clinical and economic value of CGP with the value from the perspective of multiple other stakeholders, across the entire patient journey (e.g. equity, innovation, impact on caregivers, safety to the personnel that perform the test, the potential environmental impact, and technology sustainability). MedTech Europe, a medical technology trade association, has also suggested that value methodology should be adapted to consider the value of testing for screening and early detection for all stakeholders, the clinical value of each stage of the cancer journey, and the overall value to society from more people living longer as well as better quality lives.
Collaboration should involve numerous industry partners. For example, the Precision Cancer Consortium, formed in April with founding members Bayer, GlaxoSmithKline, Novartis, and Roche, involves a dynamic partnership of pharmaceutical companies and aims to increase patient access to precision diagnostics using CGP. Furthermore, two special interest groups in the International Society for Pharmacoeconomics and Outcomes Research have recently announced a joint project to improve the transparency and evidence basis upon which CGP is evaluated and reimbursed accordingly. More initiatives such as this are critical to ensuring that all patients are able to benefit from the pioneering solutions of precision oncology.
While expanding access to CGP will be important for the foreseeable future, innovation continues, and we can expect additional high-throughput technologies such as multi-omics-based tumor and host profiling to enter clinical care in the near future. Do we expect to run into the same dilemma of limited access yet again with any new diagnostic technology entering the field, or will our current learnings from precision oncology pave the way forward?
Acknowledgments: Research support in the form of third-party medical writing assistance for this article, furnished by Stephen Salem, BSc, of Health Interactions, was provided by F. Hoffmann-La Roche Ltd.
* Multiple secondary sources were used to cross-validate information, including Trialtrove, clinicatrials.gov, EudraCT, and ChiCTR. FDA approval timeline estimate was based on Phase 3 PCD and 8 months’ review, and analysis was based on current Phase I/II, Phase II, and Phase III trials initiated before Aug. 19, 2021, with inclusion criteria requiring patient selection based on alterations in specific biomarkers. “Biomarker” was defined as any biologic molecule found in blood or tissues that has either prognostic or predictive significance in cancer treatment, and for which the effectiveness of a therapy in a patient population defined by the detection of this molecule or molecular aberration is currently being tested or has already been approved.
† Approval predictions for 2025 have a greater degree of uncertainty.
CGP, comprehensive genomic profiling; DDR, DNA damage response; FDA, US Food and Drug Administration; HRD, homologous recombination repair deficiency; MMR, DNA mismatch repair; MSI-H, microsatellite instability-high; PCD, program completion date; TMB, tumor mutational burden.