A Proposed Approach for the Management of… Leave a comment
Clot-in-transit (CIT) refers to a noticeable, free-floating thrombus inside the right-side chambers of the heart, although a thrombus in the superior vena cava (SVC) or inferior vena cava (IVC) may also fall under this heading. This type of clot is at high risk of embolization into the pulmonary artery. A right-sided heart clot is unusual, especially in the absence of a structural heart defect, in-situ catheter, or atrial fibrillation. It can be found at the bedside when we perform emergent echocardiography to assess dysfunction of the right ventricle. About 4% of patients suffering from a pulmonary embolism (PE) have a floating embolus present in the right heart, a finding that is associated with a mortality rate of more than 40% [1,2]. So, PE coupled with CIT has a higher mortality rate. Because of its high mortality rate, which ranges from 21% in 14 days to 29% in 3 months, a right-side CIT is considered a medical emergency [1]. The therapeutic options include thrombolysis, anticoagulation, catheter-directed thrombolysis, and surgical thrombectomy. The best treatment option is unknown [2,3,4].
We reviewed all the articles from the year 1982 to the present for similar studies. We used the keywords “clot in transit”, “thrombus in transit”, “treatment of the clot in transit”, “treatment of thrombus in transit”, “pulmonary embolism”, “anticoagulation”, and “Point-of-care Ultrasound (POCUS)” to find similar publications on PubMed, Google Scholar, Chest journal and The American Journal of Medicine. We screened the titles and abstracts of various studies to disregard the non-relevant studies such as those associated with coronavirus disease 2019 (COVID-19) patients and articles published in a language other than English. Inclusion criteria were the following: full-text articles published within the last 40 years, observational studies, case reports, systematic reviews, meta-analyses, narratives, and articles specifically on the diagnosis and management of CIT. Gender differences were not taken into account. We observed that there is a lack of interventional studies to favor one form of treatment strongly over the others.
Role of imaging modalities
Echocardiography
Echocardiography is still the gold standard for pulmonary embolism risk classification and detection of intracardiac thrombi, with the added benefit of determining the clot’s origin. Right heart clots can be classified into three categories based on their morphology:
Type A: Type A is the most common type. It occurs when a deep vein thrombus (DVT) breaks free and floats up to the right side of the heart. It carries the greatest risk of embolization into the pulmonary circuit. It moves freely inside the heart chambers and has an elongated, wormlike form.
Type B: The atrium or ventricle is assumed to be the source of a Type B thrombus. It is oval, has a broad base, and is adhered to the chamber wall.
Type C: Type C thrombi are uncommon, extremely migratory clots that look like cardiac myxomas [5].
Echocardiography can also be used to track right heart function after thrombolysis.
The presence of thrombi in the right heart can easily be misinterpreted as other physiological or pathological signs. One should differentiate thrombi in the right heart from congenital structures such as the atrial septal aneurysms, persistent eustachian or thebesian valves, Chiari network, and acquired pathologies such as intracardiac malignancies and vegetations [6].
If CIT is associated with acute PE (a high mortality combination), then echocardiography will show the following evidence of right heart strain: interventricular septum bowing into the left ventricle (LV), systolic dysfunction of the right ventricle (RV), and McConnell’s sign (akinesis of the free wall of the right ventricle with sparing of the apex) [7].
When transthoracic echocardiography (TTE) is non-diagnostic, transoesophageal echocardiography (TEE) is utilized to obtain a better image of the thrombus. While performing thrombectomy, real-time monitoring of the clot during atrial entry can be done using TEE. Such surveillance can decrease the possibility of collateral damage, including rupture of chordae tendineae and subsequent arrhythmia incitement. TEE monitoring also lessens the requirement for fluoroscopy, which lowers the dangers of ionizing radiation and contrast injection [8].
An invasive imaging technique called intravascular ultrasound (IVUS) can precisely detect CIT both before and during an embolectomy [9]. Another invasive imaging modality is intracardiac ultrasonography (ICE). Both of these techniques could end up being more useful. Because ICE only requires one operator, interventional cardiologists frequently use it compared to IVUS. This removes the requirement of TEE, which circumvents the possibility of tracheal trauma. Additionally, ICE cuts down on procedural and fluoroscopy times [10]. In their case report, Chen et al. documented a successful right atrial thrombus aspiration that was guided by ICE [11].Likewise, IVUS is used while performing embolectomy [9]. The limited availability and higher cost of IVUS and ICE may prevent them from being used as much as TTE or TEE, which are more widely available.
Various modalities can be used to diagnose CIT, such as echocardiography (POCUS, TTE, or TEE), Computed Tomography (CT), and Computed Tomography Angiography (CTA). The sensitivity and specificity of various imaging modalities for intracardiac thrombi have been presented in Table 1. Yastrebov et al. stated that 3-Dimensional (3D) intracardiac echocardiography gives a precise and better picture of the heart’s anatomy and pathology compared to 2-Dimensional (2D) echoes. It offers the benefit of diagnosing CIT and also providing accurate details like exact location, size, and relation to nearby structures [12]. This additional information facilitates the selection of an appropriate treatment plan. IVC, Right atrium (RA), and RV will show the presence of a filling defect on CTA if there is the presence of a CIT [13]. Timely and accurate diagnosis is so crucial for these patients. Gregory et al. report the case of a 71-year-old female with atrial fibrillation who was diagnosed with CIT by using POCUS immediately on arrival to the Emergency Department (ED). Because of the early diagnosis, the staff was already prepared for what was expected [14].
| Imaging study | Sensitivity | Specificity |
| Transthoracic echocardiography | 95% | 86% [15] |
| Computed tomography | 81% | 90% [16] |
| Computed tomography angiography | 100% | 91-100% [17] |
Table 1: Sensitivity and specificity of various imaging modalities to detect intracardiac thrombi
The prevalence of CIT is estimated to be between 4 and 18 percent; however, as POCUS becomes more prevalent, this number is expected to climb. The lack of a universal treatment guideline is to blame for a 100% mortality rate if left untreated [18]. So far, we can only rely on clinical acumen and detecting CIT early by using contemporary medical technologies.
Treatment
Catheter-Based Thrombolysis
High-frequency ultrasound exposure near the clot’s surface or percutaneous catheter-directed thrombolysis, endovascular mechanical thrombectomy using a capture device (such as a basket) with fragmentation, as well as endovascular suction of the clot directly from the pulmonary arteries, ventricle, or atrium are just a few examples of approaches to intervention.
Advantage: These operations provide a high success rate and reduce the severity of major and minor bleeding [19].
Disadvantage: Risk of thrombus dislodgement; bulky thrombi are usually resistant to thrombolysis.
Two thrombectomy systems are presented in Table 2.
| Device Name | Description | Comments | Literature search |
| FlowTriever device | A mechanical and suction device. | Its lack of need for extracorporeal filtration or cardiopulmonary bypass is an advantage. | Few case reports have shown good results for treating CIT [19,20] |
| AngioVac device | A thrombectomy tool with FDA approval can aspirate intravascular debris, including tumors, foreign substances, and thrombus. | The device’s rigidity, mobility, and the possibility of RV perforation are major technical challenges. It can’t be used in noncardiovascular centers because it requires an ECMO setup and a perfusionist who isn’t always accessible. |
Table 2: Thrombectomy systems
ECMO: extracorporeal membrane oxygenation; RV: right ventricle; CIT: clot-in-transit
In a literature review, Worku B. et al stated that AngioVac was an effective alternative to performing surgical thrombectomy for patients who presented with intracardiac or iliocaval thrombi with a success rate of more than 80% [21]. On the other hand, Bayona M et al. proposed that using FlowTriever would be better than using AngioVac due to no need for extracorporeal perfusion. It can be a preferred method for the treatment of CIT and PE, especially for people in whom thrombolysis is contraindicated [22].
Type A thrombus has a high risk for embolization, which is why they must be treated aggressively with thrombolytics and surgical embolectomy. Type B is at low risk for embolization and can be medically managed. Whereas for Type C, there is no strong literature supporting treatment, but Reddy et al. used Generation 3 AngioVac system to remove a Type C right atrial thrombi in a 25-year-old male patient who was in remission for acute myeloid leukemia. The patient had denied surgical thrombectomy and the systemic coagulation was failing to show any improvements [23]. When the clot was removed it was noticed that it was calcified, but AngioVac is used to remove soft, fresh clots [24].
Akhmerov A et al. discussed a retrospective study with 13 patients treated with AngioVac with variable indications and they found that 77% of patients made it to discharge from the hospital. Eight patients were suffering from right ventricular dysfunction, but after the procedure, 11 out of the 13 patients had markedly improved RV functions [25]. As we know that CIT causes right ventricular dysfunction, especially when associated with PE [26], AngioVac can serve as an extraordinary treatment not only to remove CIT but also to reduce the complications from CIT. But in a project by Rose et al., 177 cases of right heart thromboemboli were studied. Subjects were categorized into four different groups respective to the treatment modality and compared with mortality rates. Mortality was 100% without any treatment, 28.6% with anticoagulation, 23.8% with surgical embolectomy, and 11.3% with thrombolysis [27]. Given the lowest mortality with thrombolysis, it seems a great option but its use is concerning in patients with a bleeding disorder or history of head trauma. So, a trial for AngioVac can be performed as an alternative in those patients. We propose that AngioVac would serve as a breakthrough model for the treatment of all types of CIT.
In an interventional study performed by Liu B et al. on 20 patients with DVT and acute PE, it was found that a combination of catheter-directed thrombolysis (CDT) and percutaneous mechanical thrombectomy (PMT) had a success rate of 100%. During their hospitalization, there were no drastic events. Only four patients had to undergo iliac vein stent placement due to iliac vein compression syndrome. The patients were followed up and no event of recurrence was noticed for 16.5+/-6.8 months [28]. Interventional studies should be performed adopting a similar strategy for a CIT as well.
Surgical Embolectomy
Depending on the size of the CIT, more invasive procedures may be required. Yang et al demonstrate a case report where they performed a surgical resection by lower mini-sternotomy due to the large size of the CIT [29]. It consists of surgically opening the right atria and/or pulmonary trunk and manually extracting the thrombus. It’s coupled with a full cardiopulmonary bypass.
Advantage: If right-to-left heart communication is present, surgical embolectomy provides a more decisive treatment and may provide a chance to close the communication in the same sitting. For patients who are hemodynamically unstable, it is a favored treatment option [27,30].
Disadvantage: It necessitates substantial heart surgery as well as cardiopulmonary bypass. The technique cannot be performed in hospitals with minimal surgical infrastructure. Prolonged time-to-treatment, general anesthesia, cardiac bypass, secondary infection, and failure to eradicate concurrent pulmonary emboli outside the central pulmonary arteries are all possible risks [31,32,33].
Surgical Embolectomy in the Setting of Concomitant CIT and PE
Numerous studies showed that most of the time CIT was diagnosed when the patient presented with signs and symptoms suggestive of PE. Patients must be treated for CIT and PE simultaneously to avoid another episode of PE following CIT. According to a meta-analysis performed by selecting six cohort studies that consisted of 15,220 patients who suffered from symptomatic PE that was acute in onset and also had a right heart thrombus that was detected by echo, it was proved that there was a 3.0 times threat of poor short-term prognosis in patients having right heart thrombi (RHT) along with PE than PE alone [34]. Not only does it increase mortality, but it also changes the treatment plan. In a case report by Medina MA et al. of CT Scan diagnosed PE along with thrombus of the left subclavian artery, emergent surgical pulmonary embolectomy was performed for CIT with acute massive pulmonary embolism. They found that the technique is associated with lower risks and has excellent results [35].
Anticoagulants: The advantage of anticoagulants is the ease and rapidity of administration. They can be reserved for patients in whom surgery is contraindicated (e.g., advanced age, progressive cancer, recent brain surgery, gynecological-obstetric bleeding, and stroke). The disadvantage is that pulmonary or systemic ischemia events may occur as a result of bleeding problems or thrombus fragmentation. They only prevent clot proliferation; they do not affect a pre-existing clot.
Anticoagulants in the Setting of Concomitant CIT and PE
An evidence-based study was performed by Barrios et al. on 325 patients having RHT and PE. Patients received anticoagulation or anticoagulation + reperfusion. They found that there was not much statistical difference between both groups with regard to all-cause mortality and episodes of major bleeding. But in the follow-up period, it was found that 6.2% had a recurrence in the reperfusion group [36]. A case report by Mardinger demonstrated a report of a 32-year-old female suffering from a CIT and a PE postoperatively, who was successfully treated with only half a dose of anticoagulation in combination with intravenous (IV) unfractionated heparin [37].
Systemic Thrombolysis: The advantages are – it improves left and right ventricular output by increasing right ventricular function and minimizing RV-LV dependency. It also reduces pulmonary hypertension and improves thrombus lysis and pulmonary reperfusion [38]. Furthermore, it has the ability to dissolve clots in three separate places simultaneously: pulmonary embolus, intracardiac thrombus, and venous thrombosis. Lastly, it’s a straightforward, quick, and universally applicable intervention that can be performed at the patient’s bedside [39]. Additionally, in a crashing, near-to-death patient, or during cardiac arrest, this may be the only option. The disadvantage is that there is a risk of embolizing in the lungs after the clot breaks free, especially when there is a thrombus already present in the lungs and bleeding.
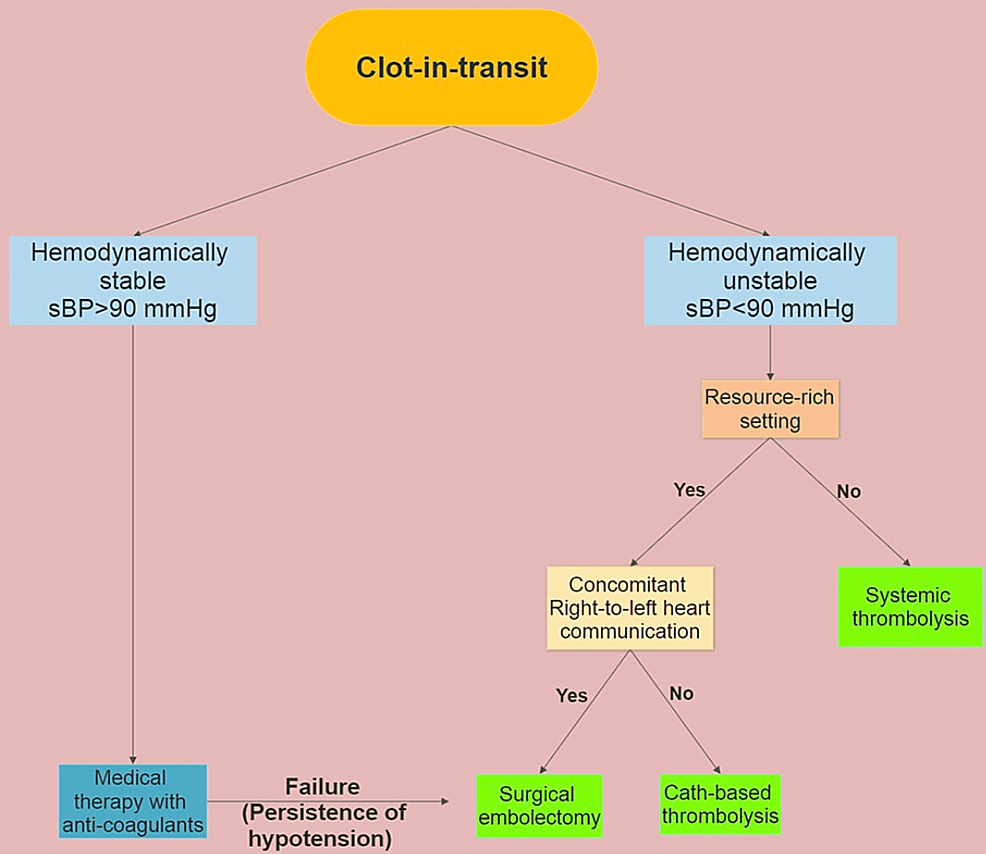
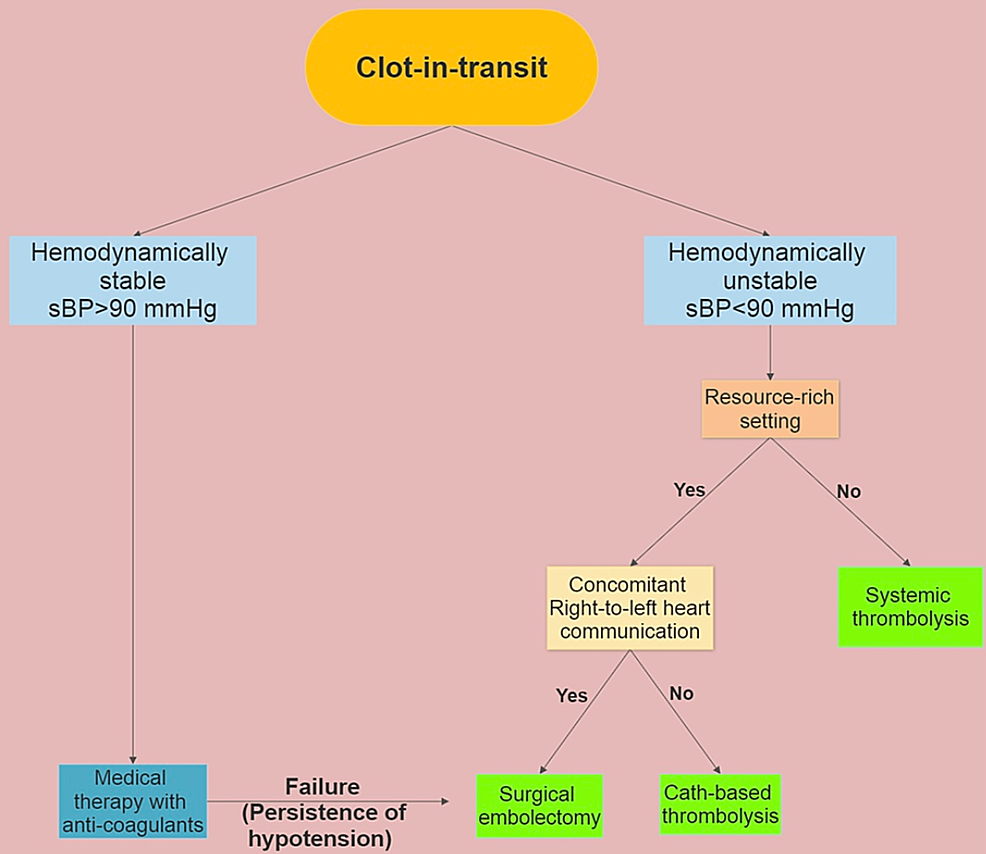
Management plans should be undertaken based on individual cases until more definitive data is available, considering complicating conditions such as hemodynamic instability, right heart function, Patent Foramen Ovale (PFO), and malignancy. No randomized controlled trials have directly compared the therapy modalities; hence, the best therapeutic approach is still up for debate. We propose here an algorithm that depicts our approach for the treatment of CIT (Figure 1).
Figure 1: Suggested algorithm for the treatment of clot-in-transit
Resource-rich setting: An operating room with cardiopulmonary bypass setup and experienced surgeon available round-the-clock.